CHAPTER 5 DIFFUSION Material transport by atomic motion
CHAPTER 5: DIFFUSION • Material transport by atomic motion Issues to consider: • Types • Predicting the rate of diffusion • Factors that influence diffusion • Importance of diffusion in materials processing
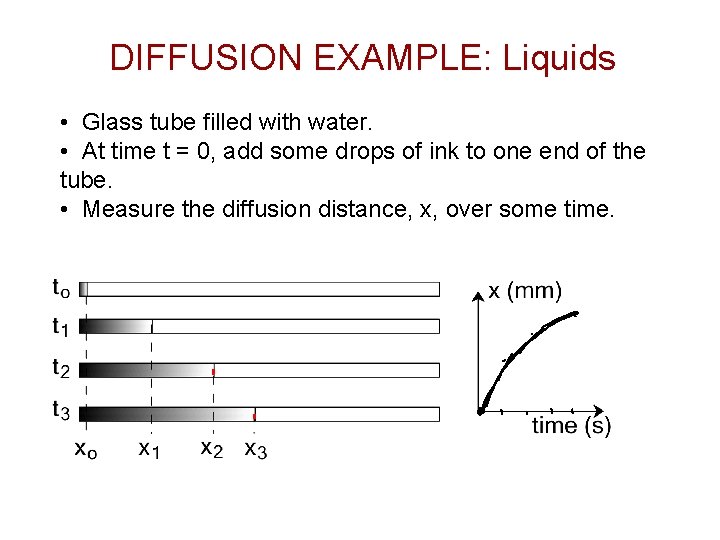
DIFFUSION EXAMPLE: Liquids • Glass tube filled with water. • At time t = 0, add some drops of ink to one end of the tube. • Measure the diffusion distance, x, over some time.
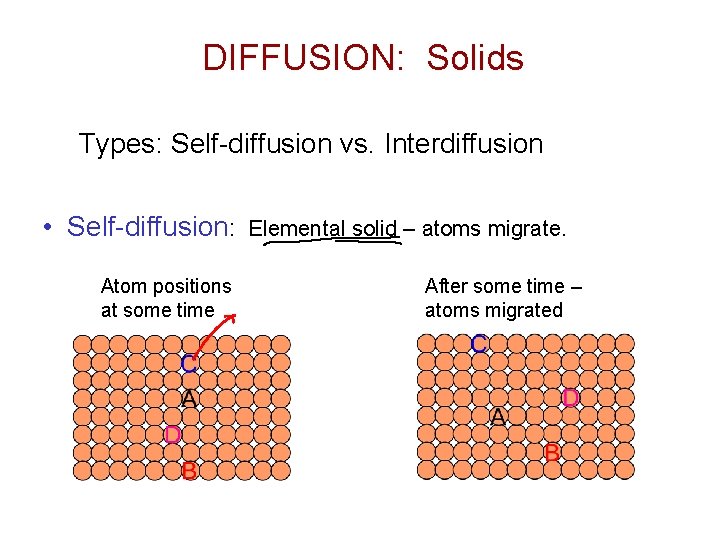
DIFFUSION: Solids Types: Self-diffusion vs. Interdiffusion • Self-diffusion: Atom positions at some time Elemental solid – atoms migrate. After some time – atoms migrated
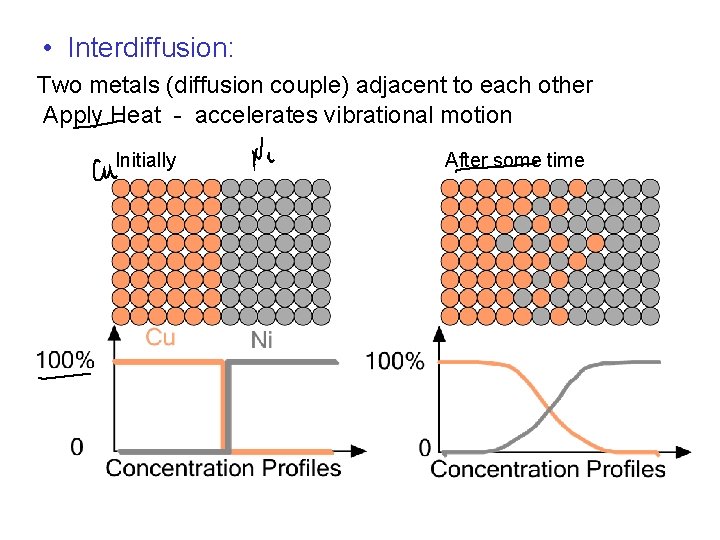
• Interdiffusion: Two metals (diffusion couple) adjacent to each other Apply Heat - accelerates vibrational motion Initially After some time
DIFFUSION MECHANISMS Vacancy Diffusion: • atoms move to an adjacent vacant lattice site • for both self- and interdiffusion (substitutional impurities) • rate depends on: --number of vacancies --activation energy
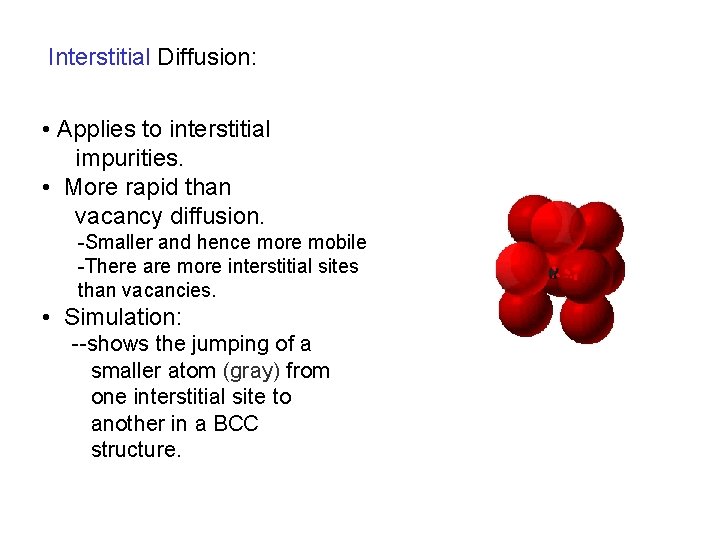
Interstitial Diffusion: • Applies to interstitial impurities. • More rapid than vacancy diffusion. -Smaller and hence more mobile -There are more interstitial sites than vacancies. • Simulation: --shows the jumping of a smaller atom (gray) from one interstitial site to another in a BCC structure.
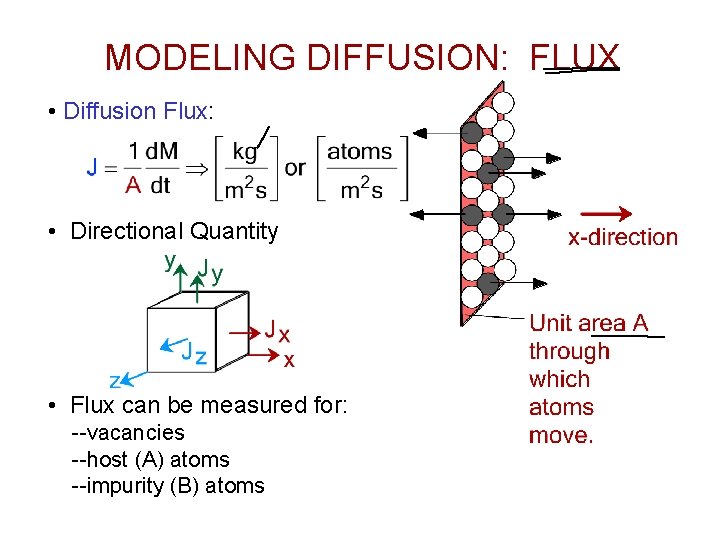
MODELING DIFFUSION: FLUX • Diffusion Flux: • Directional Quantity • Flux can be measured for: --vacancies --host (A) atoms --impurity (B) atoms
![Fick’s First Law • Concentration Profile, C(x): [kg/m 3] • Fick's First Law: • Fick’s First Law • Concentration Profile, C(x): [kg/m 3] • Fick's First Law: •](http://slidetodoc.com/presentation_image_h/8a4ebde500fbddb638a17a1bdd749f92/image-8.jpg)
Fick’s First Law • Concentration Profile, C(x): [kg/m 3] • Fick's First Law: • Negative sign – diffusion from higher to lower concentration. • The steeper the concentration profile, the greater the flux.
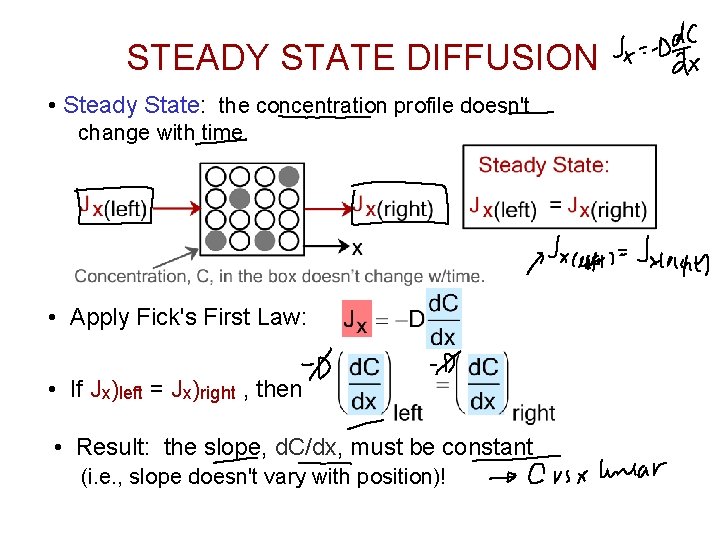
STEADY STATE DIFFUSION • Steady State: the concentration profile doesn't change with time. • Apply Fick's First Law: • If Jx)left = Jx)right , then • Result: the slope, d. C/dx, must be constant (i. e. , slope doesn't vary with position)!
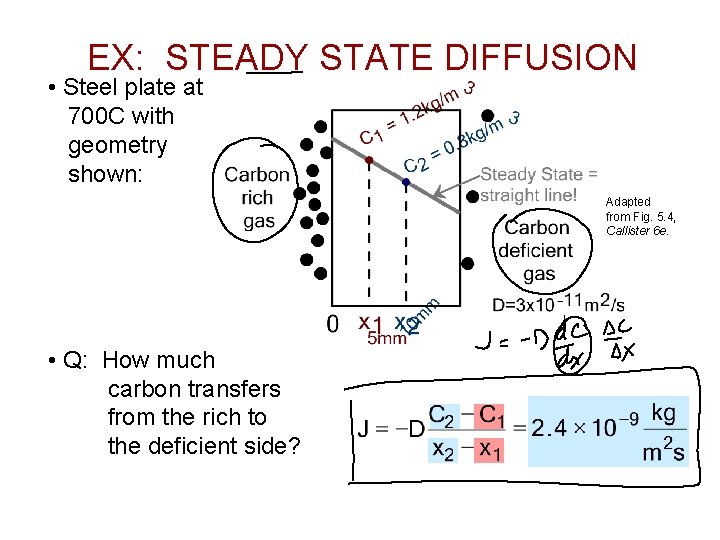
EX: STEADY STATE DIFFUSION • Steel plate at 700 C with geometry shown: Adapted from Fig. 5. 4, Callister 6 e. • Q: How much carbon transfers from the rich to the deficient side?
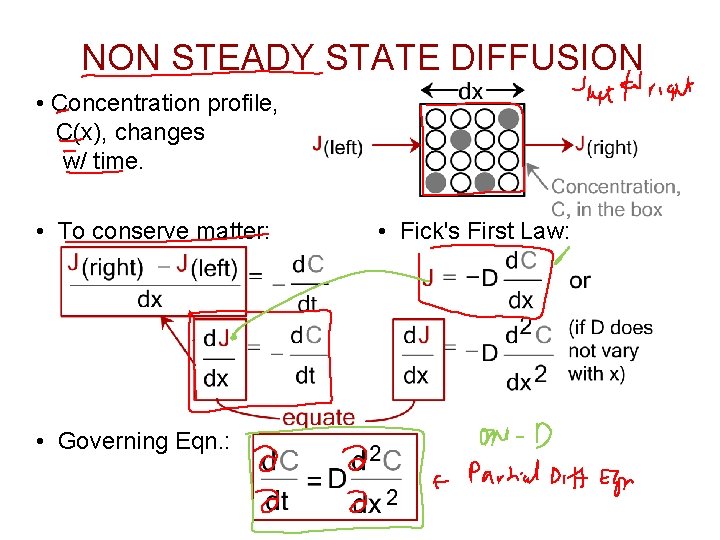
NON STEADY STATE DIFFUSION • Concentration profile, C(x), changes w/ time. • To conserve matter: • Governing Eqn. : • Fick's First Law:
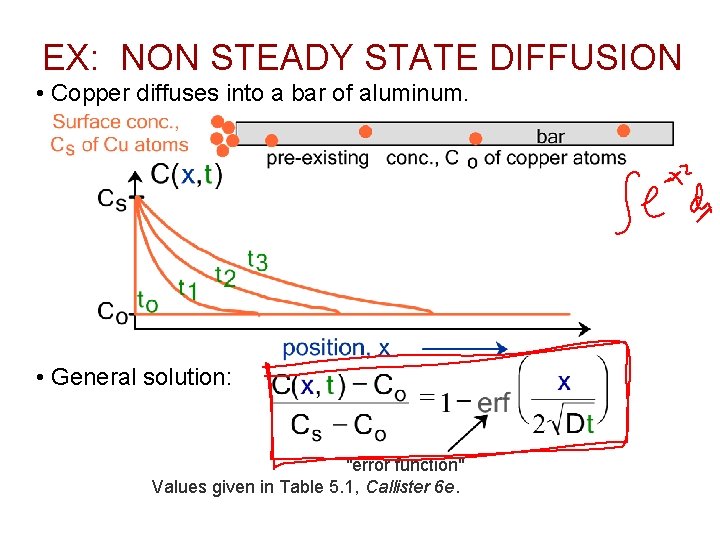
EX: NON STEADY STATE DIFFUSION • Copper diffuses into a bar of aluminum. • General solution: "error function" Values given in Table 5. 1, Callister 6 e.
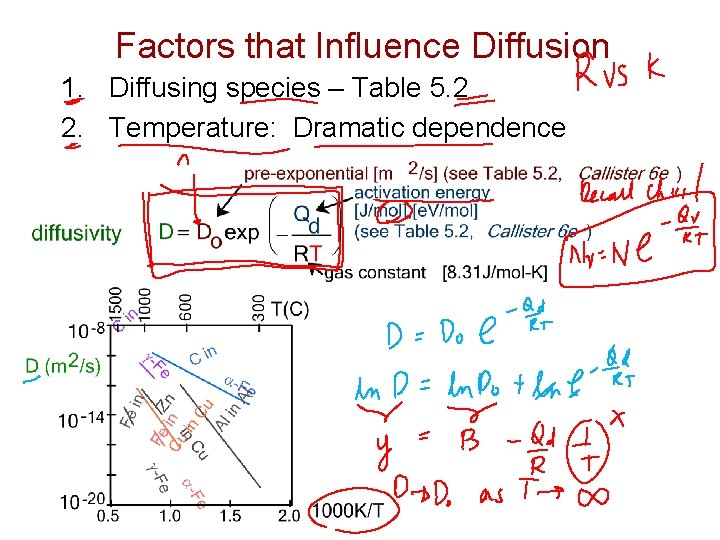
Factors that Influence Diffusion 1. Diffusing species – Table 5. 2 2. Temperature: Dramatic dependence
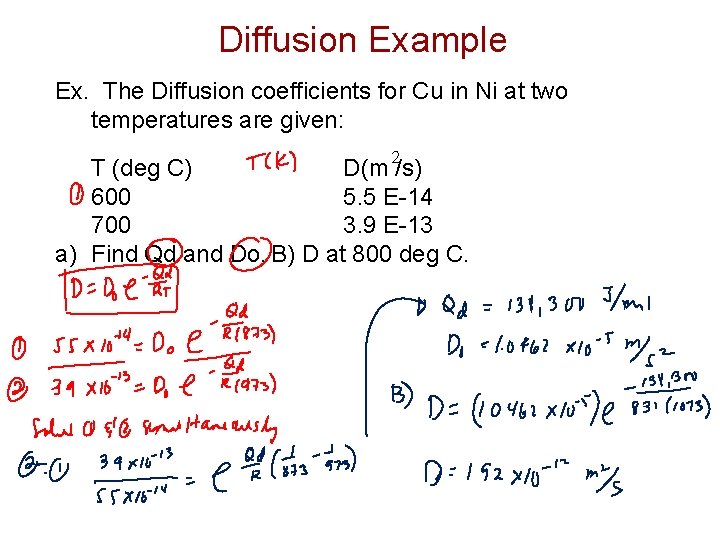
Diffusion Example Ex. The Diffusion coefficients for Cu in Ni at two temperatures are given: 2 T (deg C) D(m /s) 600 5. 5 E-14 700 3. 9 E-13 a) Find Qd and Do. B) D at 800 deg C.
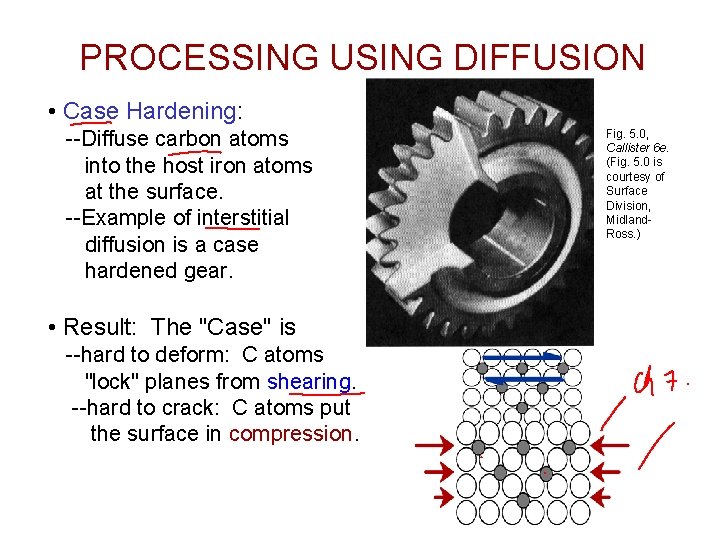
PROCESSING USING DIFFUSION • Case Hardening: --Diffuse carbon atoms into the host iron atoms at the surface. --Example of interstitial diffusion is a case hardened gear. • Result: The "Case" is --hard to deform: C atoms "lock" planes from shearing. --hard to crack: C atoms put the surface in compression. Fig. 5. 0, Callister 6 e. (Fig. 5. 0 is courtesy of Surface Division, Midland. Ross. )
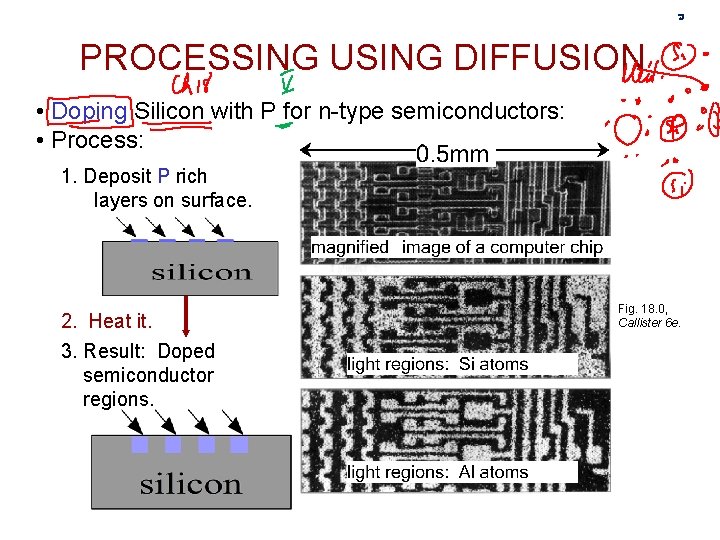
PROCESSING USING DIFFUSION • Doping Silicon with P for n-type semiconductors: • Process: 1. Deposit P rich layers on surface. 2. Heat it. 3. Result: Doped semiconductor regions. Fig. 18. 0, Callister 6 e.
- Slides: 16