CHAPTER 5 DIFFUSION Diffusion It is the phenomenon

- Slides: 8

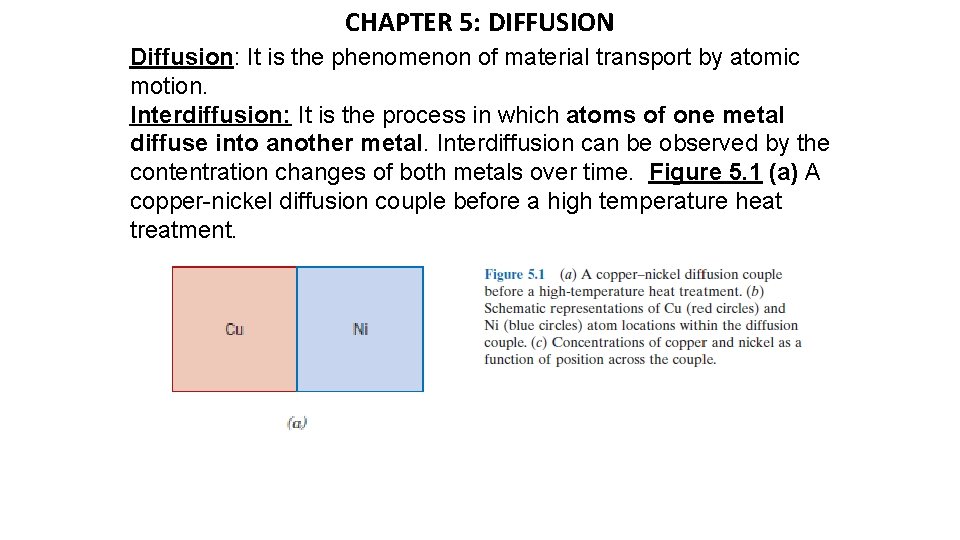
CHAPTER 5: DIFFUSION Diffusion: It is the phenomenon of material transport by atomic motion. Interdiffusion: It is the process in which atoms of one metal diffuse into another metal. Interdiffusion can be observed by the contentration changes of both metals over time. Figure 5. 1 (a) A copper-nickel diffusion couple before a high temperature heat treatment.

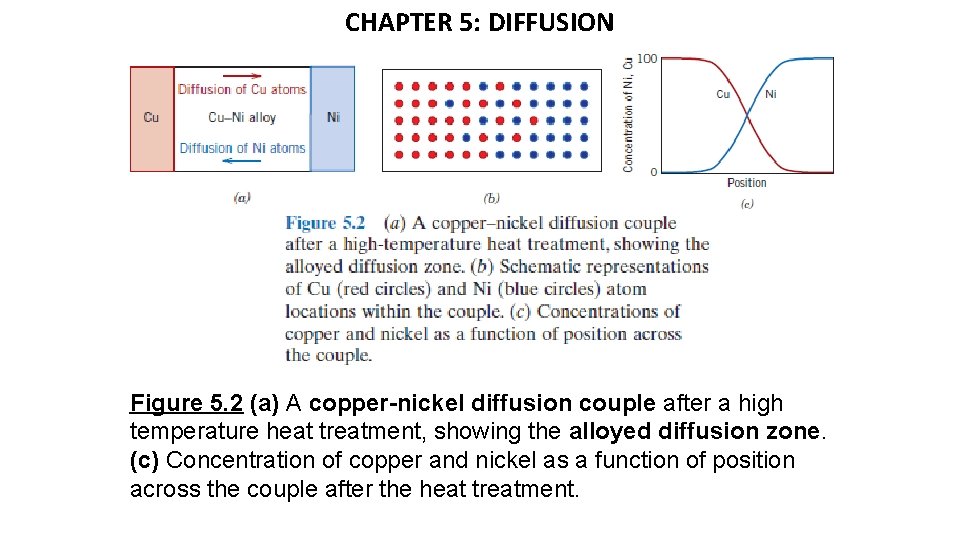
CHAPTER 5: DIFFUSION Figure 5. 2 (a) A copper-nickel diffusion couple after a high temperature heat treatment, showing the alloyed diffusion zone. (c) Concentration of copper and nickel as a function of position across the couple after the heat treatment.

CHAPTER 5: DIFFUSION Self-diffusion: If diffusion of atoms occurs in pure metals, this diffusion is called self-diffusion. Self-diffusion cannot be observed by concentration changes because there is only one type of atom in a pure metal which diffuses in the metal.

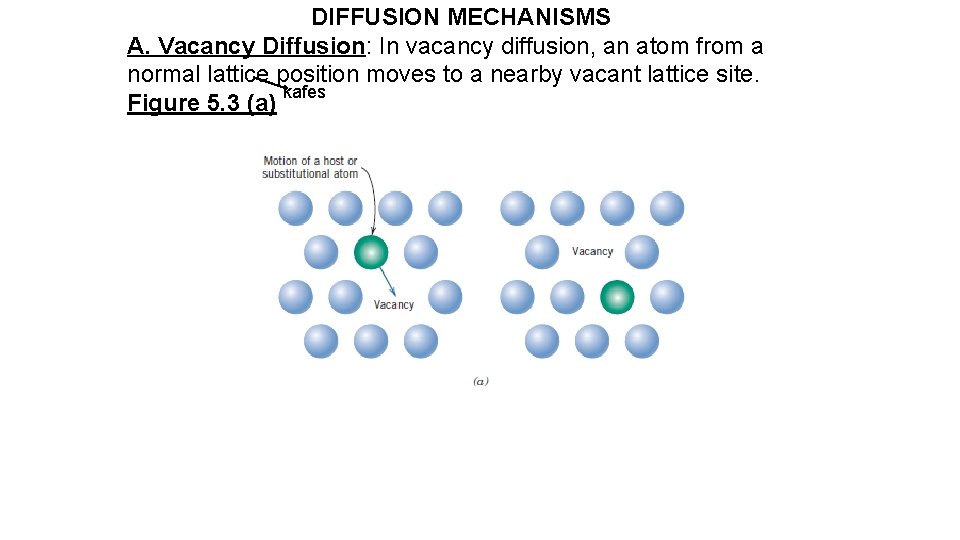
DIFFUSION MECHANISMS A. Vacancy Diffusion: In vacancy diffusion, an atom from a normal lattice position moves to a nearby vacant lattice site. kafes Figure 5. 3 (a)

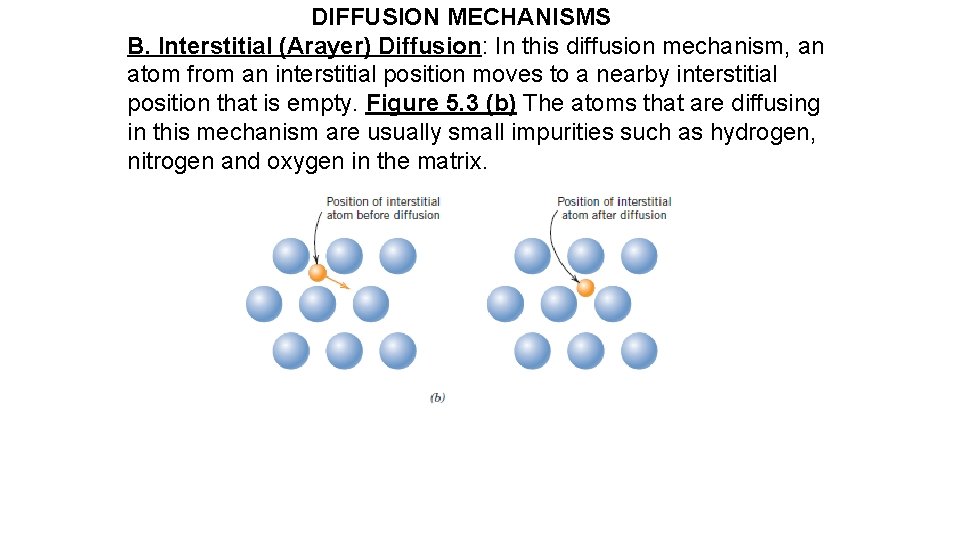
DIFFUSION MECHANISMS B. Interstitial (Arayer) Diffusion: In this diffusion mechanism, an atom from an interstitial position moves to a nearby interstitial position that is empty. Figure 5. 3 (b) The atoms that are diffusing in this mechanism are usually small impurities such as hydrogen, nitrogen and oxygen in the matrix.

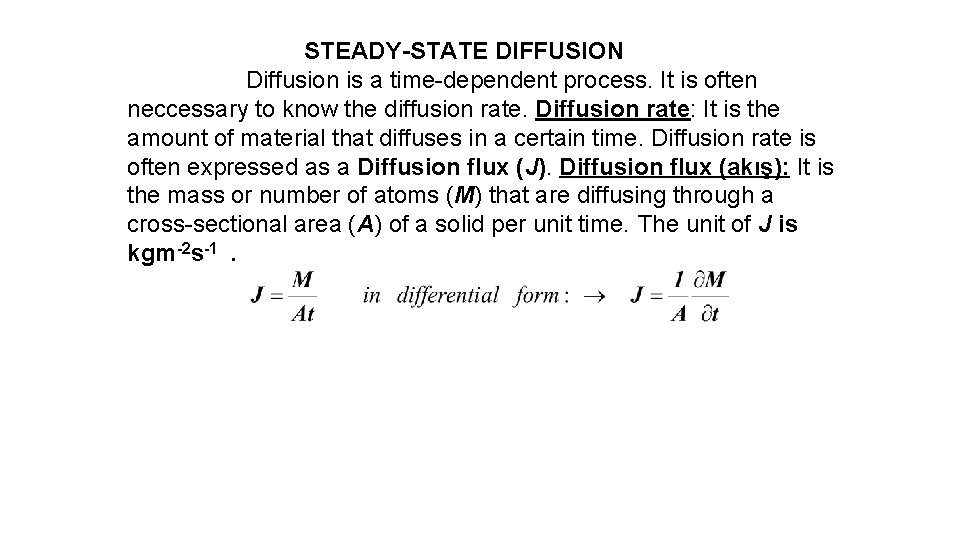
STEADY-STATE DIFFUSION Diffusion is a time-dependent process. It is often neccessary to know the diffusion rate. Diffusion rate: It is the amount of material that diffuses in a certain time. Diffusion rate is often expressed as a Diffusion flux (J). Diffusion flux (akış): It is the mass or number of atoms (M) that are diffusing through a cross-sectional area (A) of a solid per unit time. The unit of J is kgm-2 s-1.

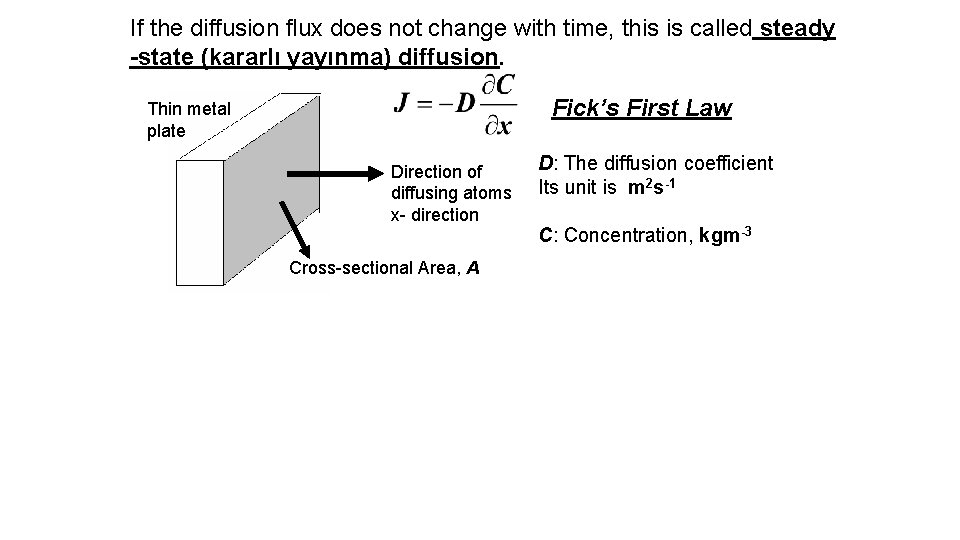
If the diffusion flux does not change with time, this is called steady -state (kararlı yayınma) diffusion. Fick’s First Law Thin metal plate Direction of diffusing atoms x- direction Cross-sectional Area, A D: The diffusion coefficient Its unit is m 2 s-1 C: Concentration, kgm-3

FACTORS THAT INFLUENCE DIFFUSION 1. Diffusing atoms: Different materials have different Diffusion coefficients (D). If you change the type of material, then it influences diffusion. 2. Temperature: Diffusion coefficient increases with Temperature. So, if you increase the Temperature, then the diffusion coefficient increases.