Chapter 5 Chemical Reactions The Mole The Mole




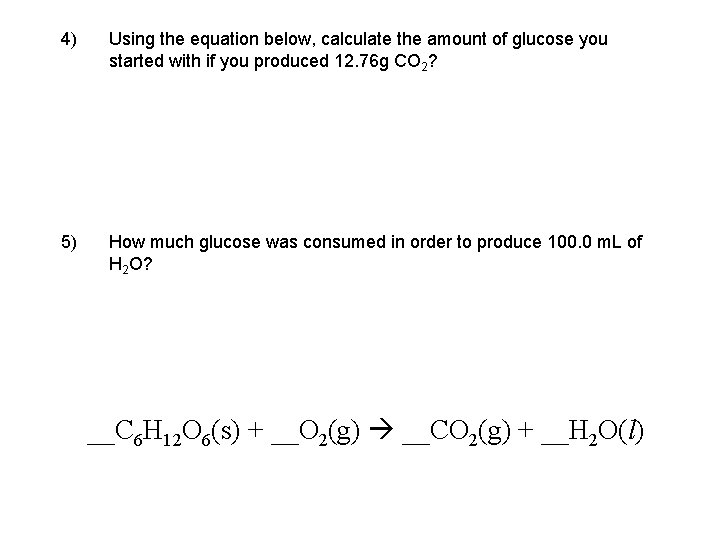




- Slides: 35

Chapter 5 Chemical Reactions

The Mole

The Mole • 1 mole = 6. 022 x 1023 things – Avogadro’s number • The number of carbon atoms in 12 g of C-12 • Abbreviation: “mol” How much does this weigh?

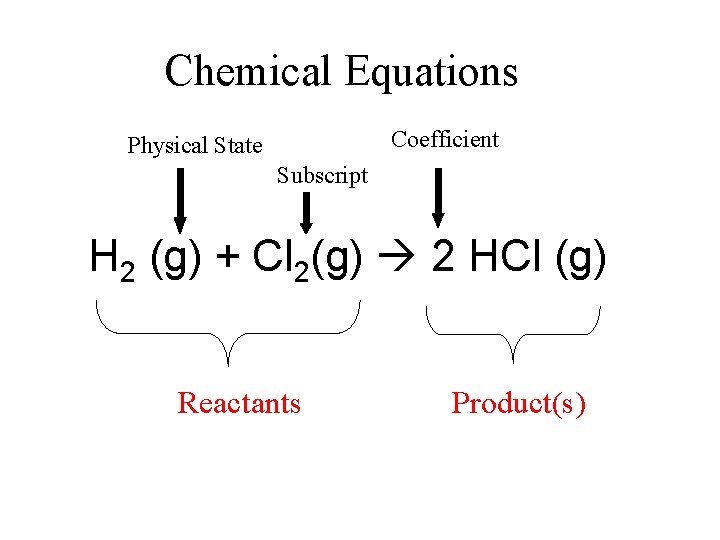
Chemical Equations Coefficient Physical State Subscript H 2 (g) + Cl 2(g) 2 HCl (g) Reactants Product(s)

Balancing Chemical Equations Zn(s) + HCl(aq) H 2(g) + Zn. Cl 2(aq) 1) 2) 3) 4) Write the unbalanced equation Balance the atoms of one element Choose another element and balance it Continue until all elements have the same number of atoms on both sides of the equation 5) Check yourself

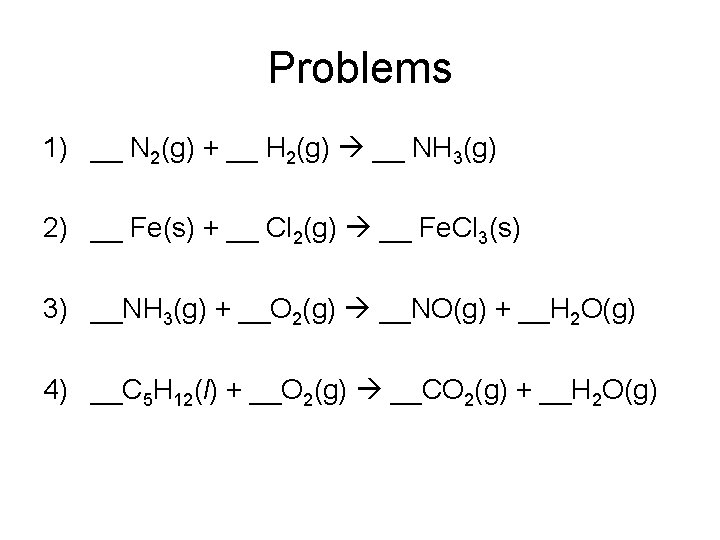
Problems 1) __ N 2(g) + __ H 2(g) __ NH 3(g) 2) __ Fe(s) + __ Cl 2(g) __ Fe. Cl 3(s) 3) __NH 3(g) + __O 2(g) __NO(g) + __H 2 O(g) 4) __C 5 H 12(l) + __O 2(g) __CO 2(g) + __H 2 O(g)

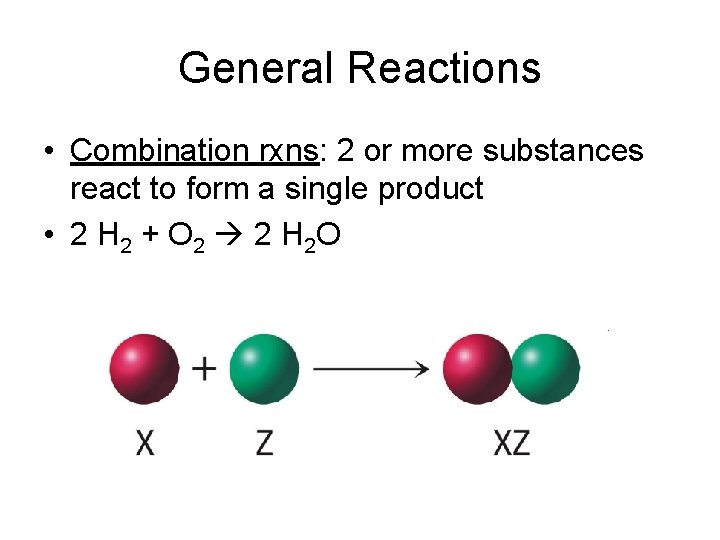
General Reactions • Combination rxns: 2 or more substances react to form a single product • 2 H 2 + O 2 2 H 2 O

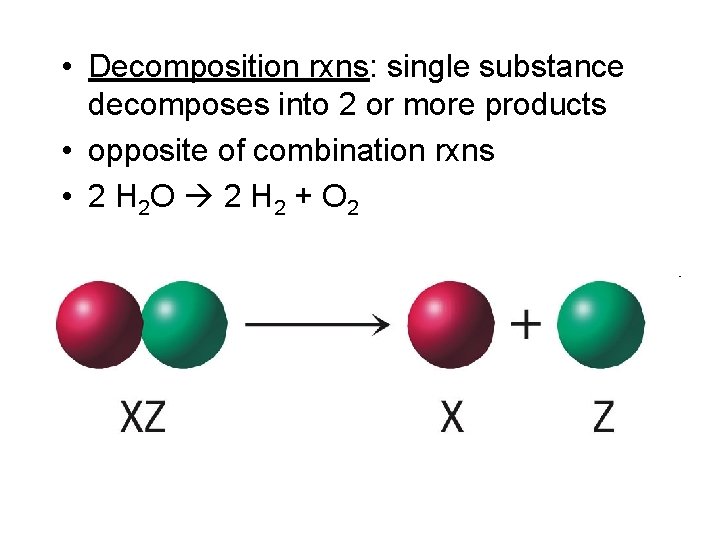
• Decomposition rxns: single substance decomposes into 2 or more products • opposite of combination rxns • 2 H 2 O 2 H 2 + O 2


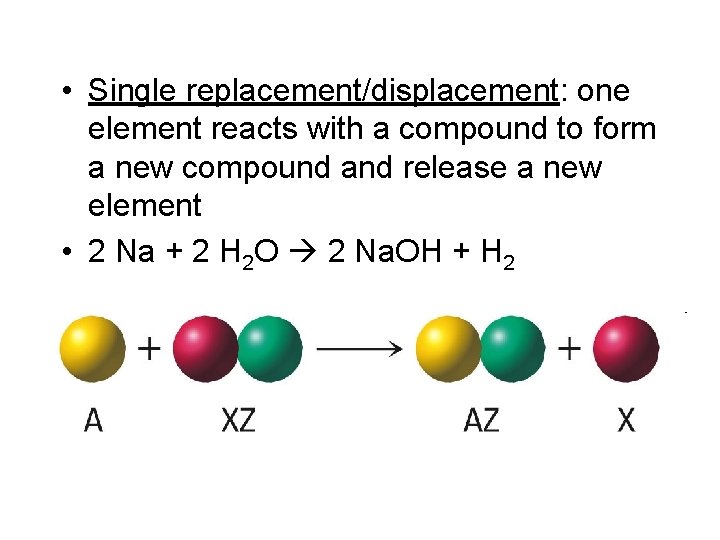
• Single replacement/displacement: one element reacts with a compound to form a new compound and release a new element • 2 Na + 2 H 2 O 2 Na. OH + H 2

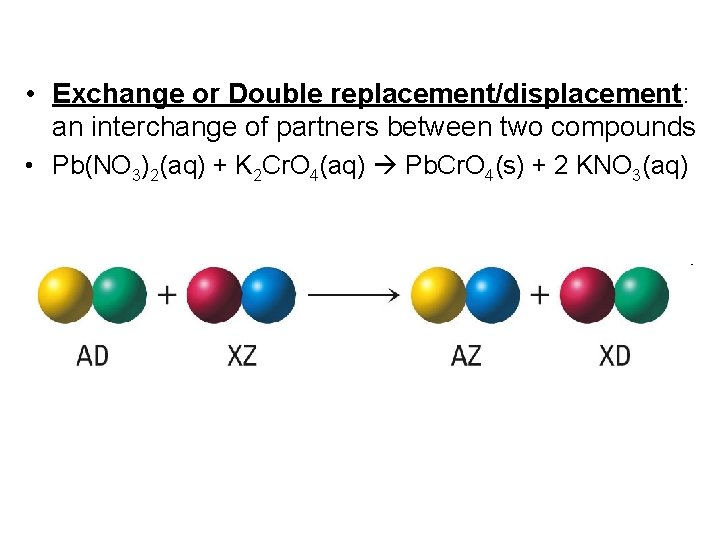
• Exchange or Double replacement/displacement: an interchange of partners between two compounds • Pb(NO 3)2(aq) + K 2 Cr. O 4(aq) Pb. Cr. O 4(s) + 2 KNO 3(aq)

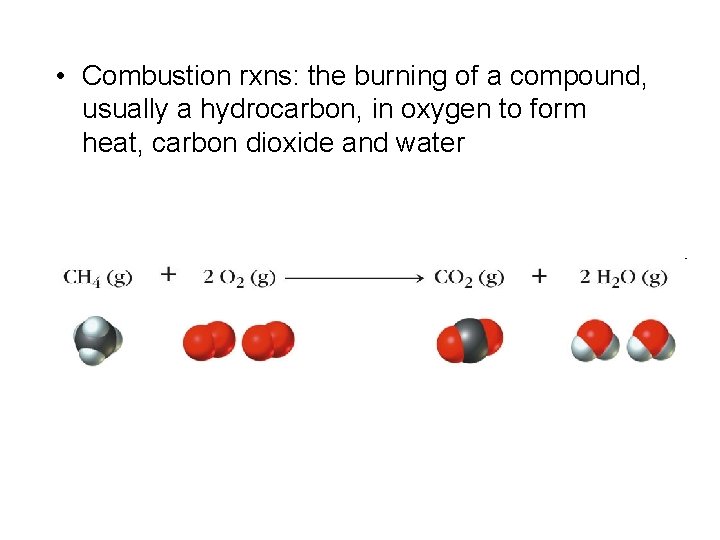
• Combustion rxns: the burning of a compound, usually a hydrocarbon, in oxygen to form heat, carbon dioxide and water

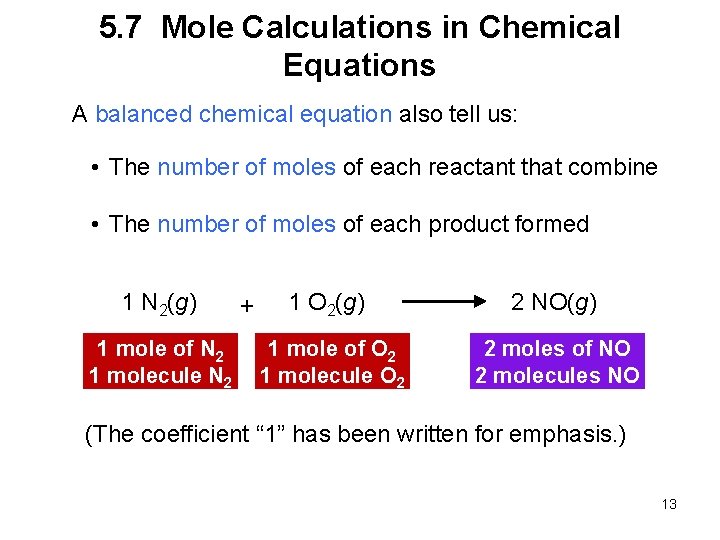
5. 7 Mole Calculations in Chemical Equations A balanced chemical equation also tell us: • The number of moles of each reactant that combine • The number of moles of each product formed 1 N 2(g) 1 mole of N 2 1 molecule N 2 + 1 O 2(g) 2 NO(g) 1 mole of O 2 1 molecule O 2 2 moles of NO 2 molecules NO (The coefficient “ 1” has been written for emphasis. ) 13

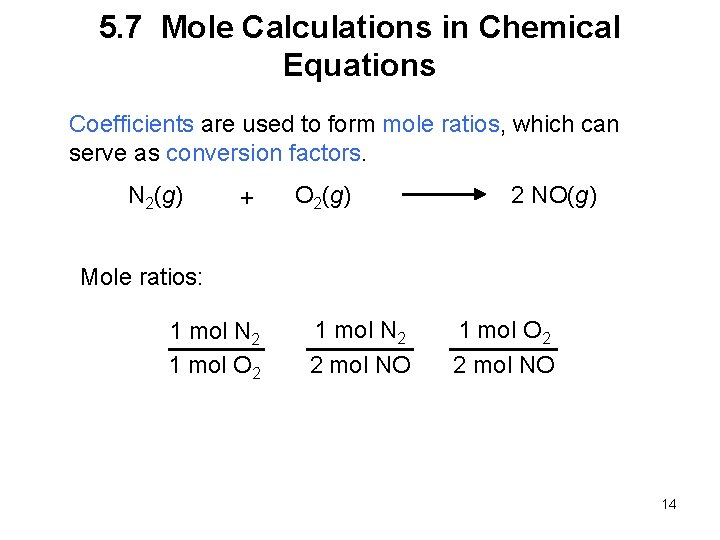
5. 7 Mole Calculations in Chemical Equations Coefficients are used to form mole ratios, which can serve as conversion factors. N 2(g) + O 2(g) 2 NO(g) Mole ratios: 1 mol N 2 1 mol O 2 1 mol N 2 2 mol NO 1 mol O 2 2 mol NO 14

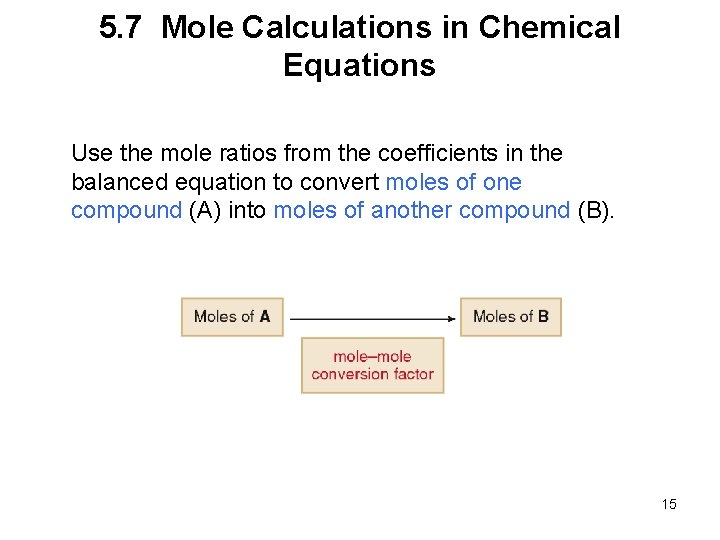
5. 7 Mole Calculations in Chemical Equations Use the mole ratios from the coefficients in the balanced equation to convert moles of one compound (A) into moles of another compound (B). 15

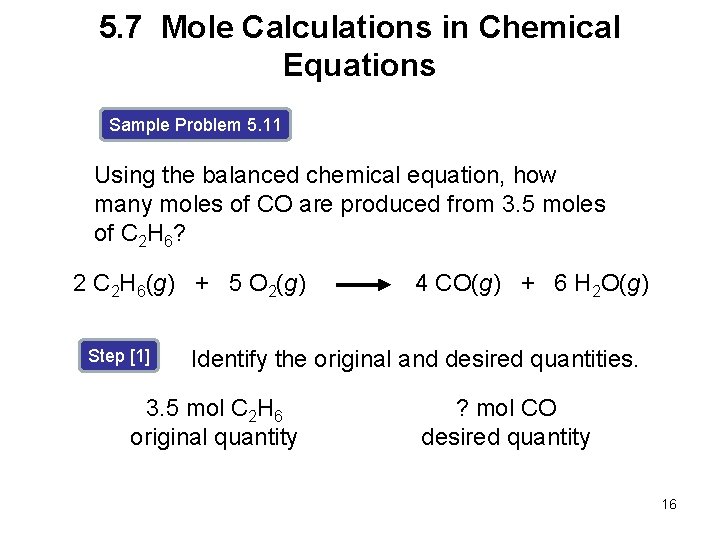
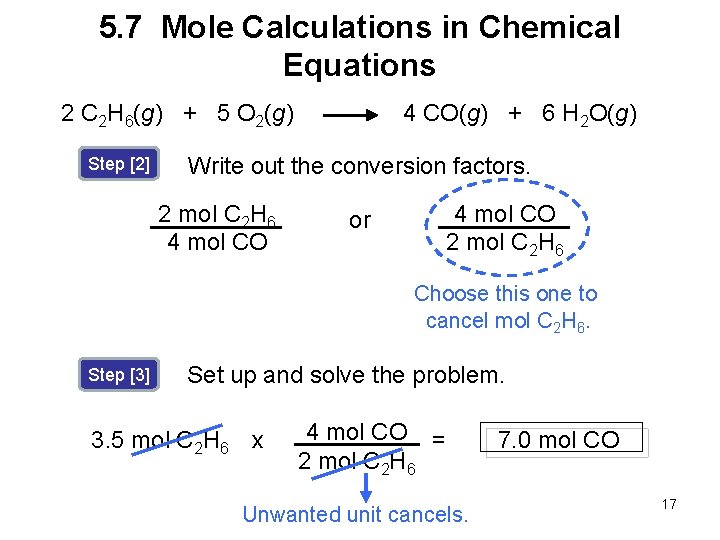
5. 7 Mole Calculations in Chemical Equations Sample Problem 5. 11 Using the balanced chemical equation, how many moles of CO are produced from 3. 5 moles of C 2 H 6? 2 C 2 H 6(g) + 5 O 2(g) Step [1] 4 CO(g) + 6 H 2 O(g) Identify the original and desired quantities. 3. 5 mol C 2 H 6 original quantity ? mol CO desired quantity 16

5. 7 Mole Calculations in Chemical Equations 4 CO(g) + 6 H 2 O(g) 2 C 2 H 6(g) + 5 O 2(g) Step [2] Write out the conversion factors. 2 mol C 2 H 6 4 mol CO 2 mol C 2 H 6 or Choose this one to cancel mol C 2 H 6. Step [3] Set up and solve the problem. 3. 5 mol C 2 H 6 x 4 mol CO = 2 mol C 2 H 6 Unwanted unit cancels. 7. 0 mol CO 17

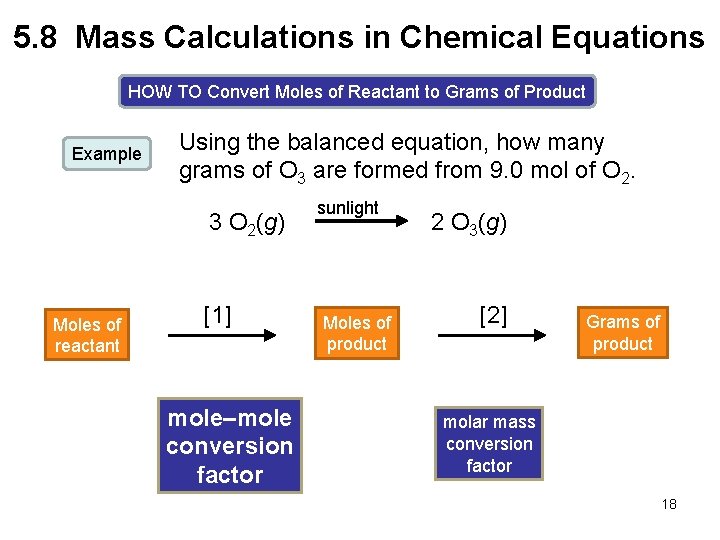
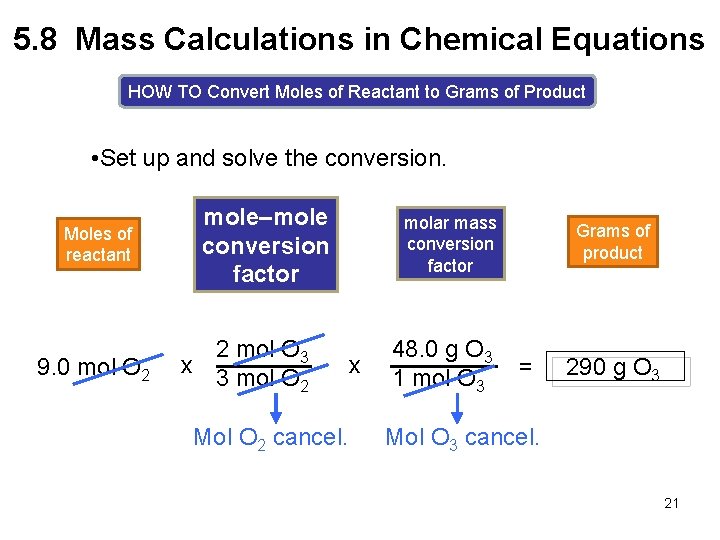
5. 8 Mass Calculations in Chemical Equations HOW TO Convert Moles of Reactant to Grams of Product Example Using the balanced equation, how many grams of O 3 are formed from 9. 0 mol of O 2. 3 O 2(g) Moles of reactant [1] mole–mole conversion factor sunlight Moles of product 2 O 3(g) [2] Grams of product molar mass conversion factor 18

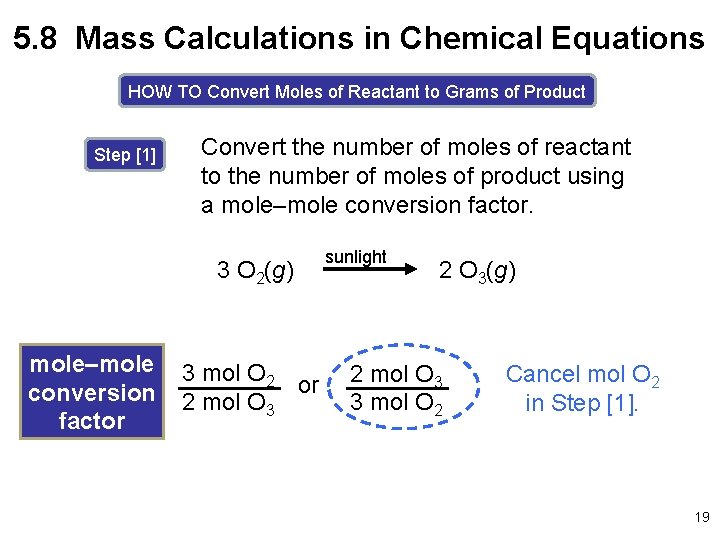
5. 8 Mass Calculations in Chemical Equations HOW TO Convert Moles of Reactant to Grams of Product Step [1] Convert the number of moles of reactant to the number of moles of product using a mole–mole conversion factor. 3 O 2(g) mole–mole conversion factor 3 mol O 2 or 2 mol O 3 sunlight 2 O 3(g) 2 mol O 3 3 mol O 2 Cancel mol O 2 in Step [1]. 19

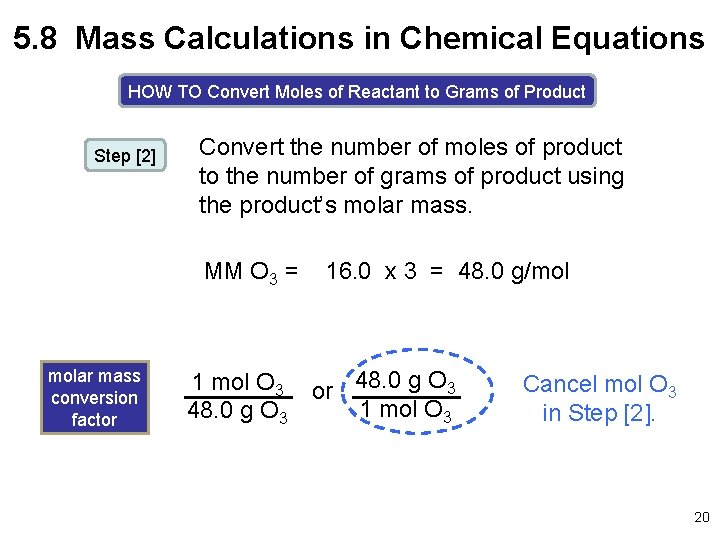
5. 8 Mass Calculations in Chemical Equations HOW TO Convert Moles of Reactant to Grams of Product Step [2] Convert the number of moles of product to the number of grams of product using the product’s molar mass. MM O 3 = molar mass conversion factor 1 mol O 3 48. 0 g O 3 16. 0 x 3 = 48. 0 g/mol or 48. 0 g O 3 1 mol O 3 Cancel mol O 3 in Step [2]. 20

5. 8 Mass Calculations in Chemical Equations HOW TO Convert Moles of Reactant to Grams of Product • Set up and solve the conversion. Moles of reactant 9. 0 mol O 2 mole–mole conversion factor 2 mol O 3 x 3 mol O 2 Mol O 2 cancel. molar mass conversion factor x 48. 0 g O 3 1 mol O 3 Grams of product = 290 g O 3 Mol O 3 cancel. 21

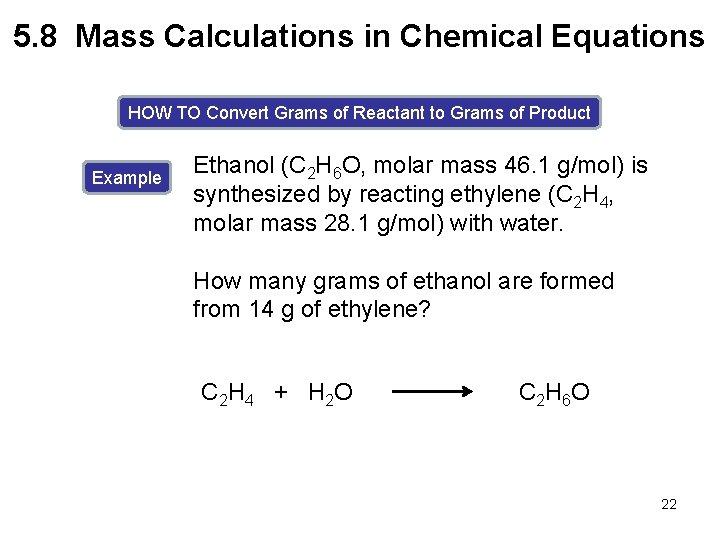
5. 8 Mass Calculations in Chemical Equations HOW TO Convert Grams of Reactant to Grams of Product Example Ethanol (C 2 H 6 O, molar mass 46. 1 g/mol) is synthesized by reacting ethylene (C 2 H 4, molar mass 28. 1 g/mol) with water. How many grams of ethanol are formed from 14 g of ethylene? C 2 H 4 + H 2 O C 2 H 6 O 22

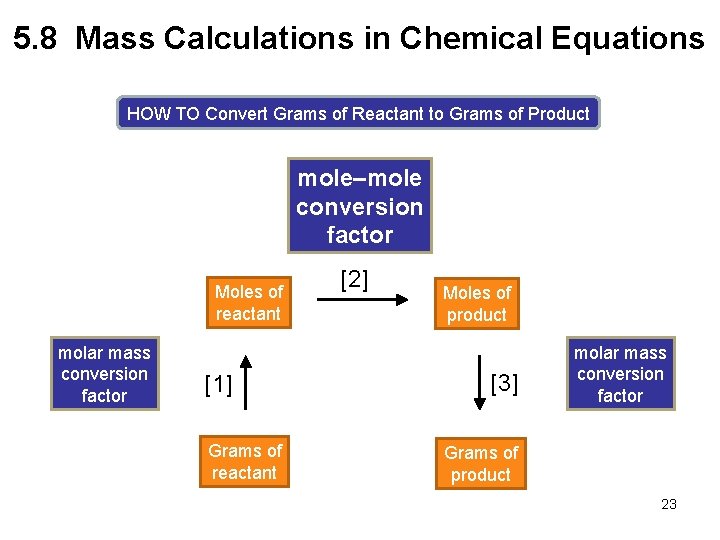
5. 8 Mass Calculations in Chemical Equations HOW TO Convert Grams of Reactant to Grams of Product mole–mole conversion factor Moles of reactant molar mass conversion factor [1] Grams of reactant [2] Moles of product [3] molar mass conversion factor Grams of product 23

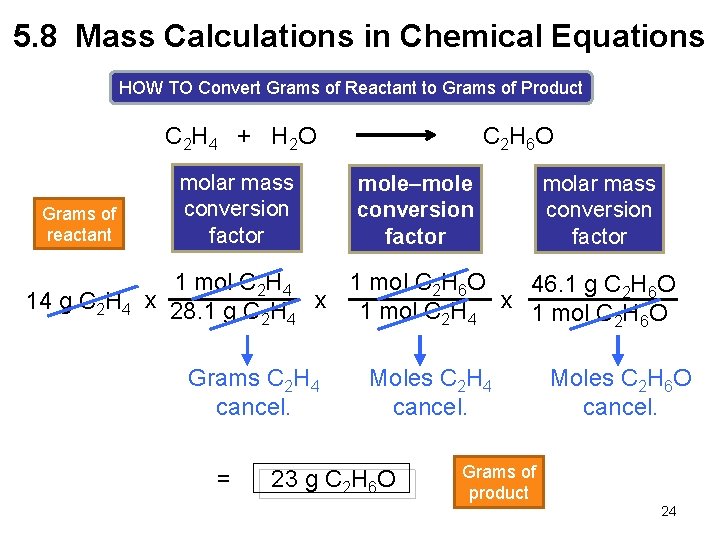
5. 8 Mass Calculations in Chemical Equations HOW TO Convert Grams of Reactant to Grams of Product C 2 H 4 + H 2 O Grams of reactant molar mass conversion factor C 2 H 6 O mole–mole conversion factor molar mass conversion factor 1 mol C 2 H 4 1 mol C 2 H 6 O 46. 1 g C 2 H 6 O 14 g C 2 H 4 x 28. 1 g C H x 1 mol C H O 2 4 2 6 Grams C 2 H 4 cancel. = Moles C 2 H 4 cancel. 23 g C 2 H 6 O Moles C 2 H 6 O cancel. Grams of product 24

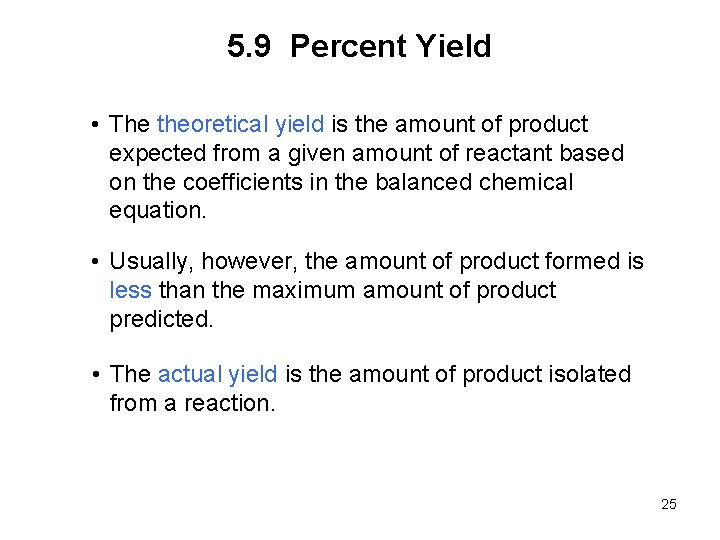
5. 9 Percent Yield • The theoretical yield is the amount of product expected from a given amount of reactant based on the coefficients in the balanced chemical equation. • Usually, however, the amount of product formed is less than the maximum amount of product predicted. • The actual yield is the amount of product isolated from a reaction. 25

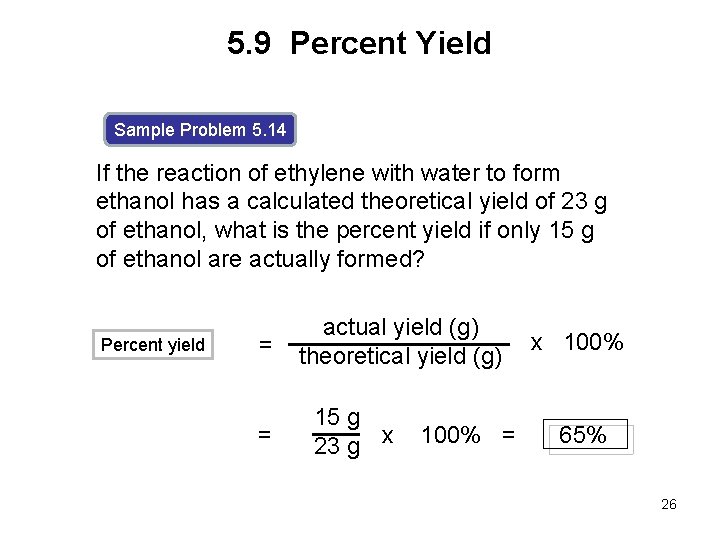
5. 9 Percent Yield Sample Problem 5. 14 If the reaction of ethylene with water to form ethanol has a calculated theoretical yield of 23 g of ethanol, what is the percent yield if only 15 g of ethanol are actually formed? Percent yield = = actual yield (g) theoretical yield (g) 15 g 23 g x 100% = x 100% 65% 26

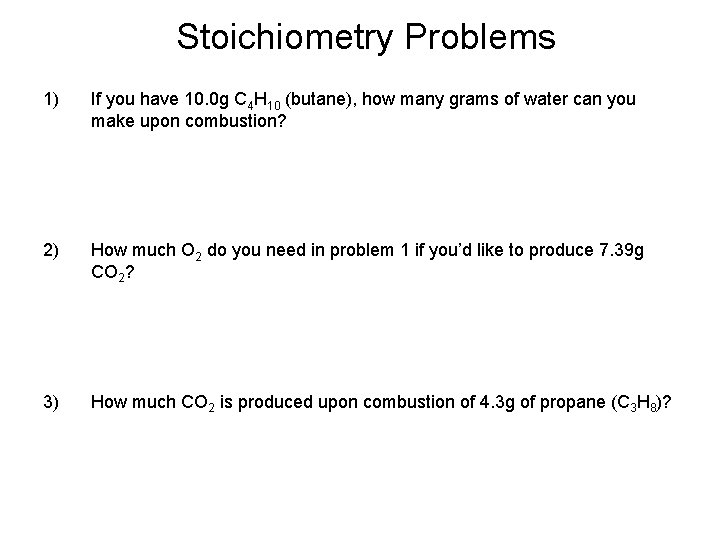
Stoichiometry Problems 1) If you have 10. 0 g C 4 H 10 (butane), how many grams of water can you make upon combustion? 2) How much O 2 do you need in problem 1 if you’d like to produce 7. 39 g CO 2? 3) How much CO 2 is produced upon combustion of 4. 3 g of propane (C 3 H 8)?
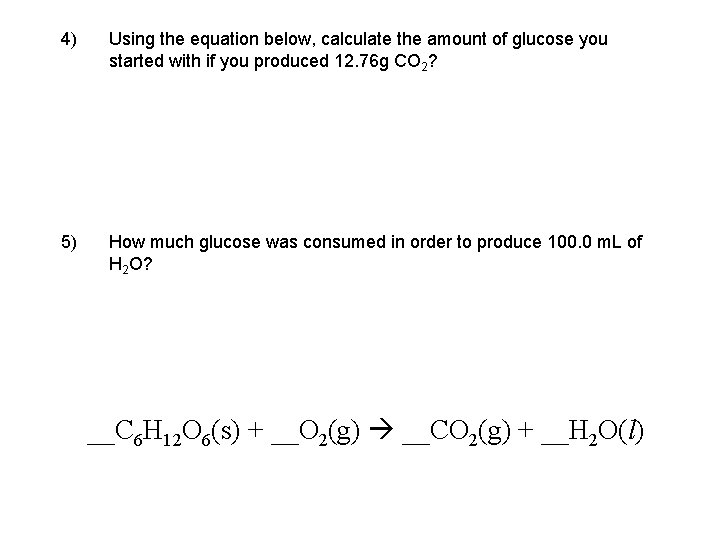
4) Using the equation below, calculate the amount of glucose you started with if you produced 12. 76 g CO 2? 5) How much glucose was consumed in order to produce 100. 0 m. L of H 2 O? __C 6 H 12 O 6(s) + __O 2(g) __CO 2(g) + __H 2 O(l)

Limiting Reagent • Limiting Reagent/Reactant/Fact or: the reactant/factor that determines the amount of product formed • Other reactants are “in excess” • Cheaper reactants are usually in excess

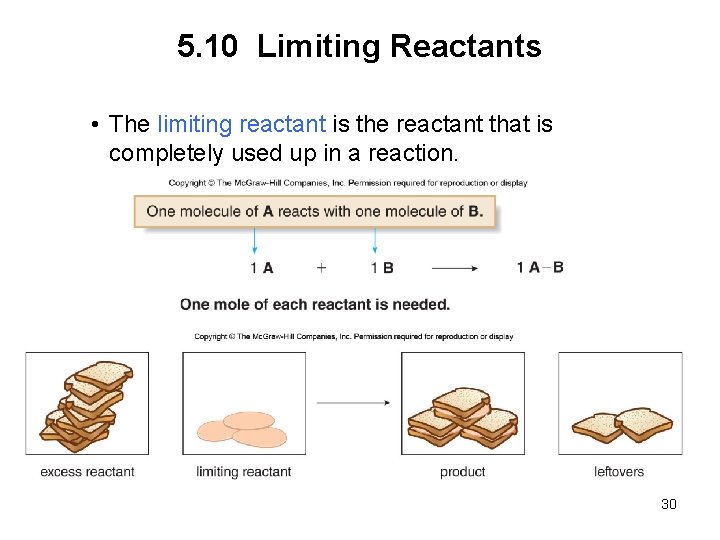
5. 10 Limiting Reactants • The limiting reactant is the reactant that is completely used up in a reaction. 30

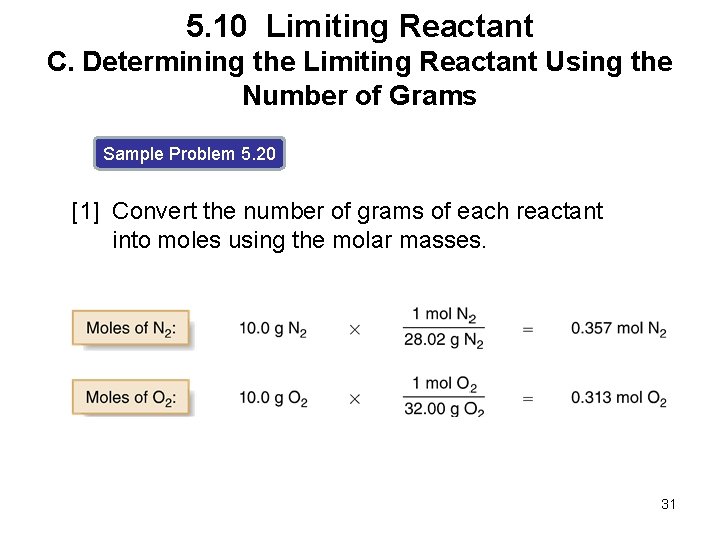
5. 10 Limiting Reactant C. Determining the Limiting Reactant Using the Number of Grams Sample Problem 5. 20 [1] Convert the number of grams of each reactant into moles using the molar masses. 31

5. 10 Limiting Reactant C. Determining the Limiting Reactant Using the Number of Grams Sample Problem 5. 20 [2] Determine the limiting reactant by choosing N 2 as the original quantity and converting to mol O 2. mole–mole Conversion factor 0. 357 mol N 2 x 1 mol O 2 1 mol N 2 = 0. 357 mol O 2 The amount of O 2 we started with (0. 313 mol) is less than the amount we would need (0. 357 mol) so O 2 is the limiting reagent. 32

Problems 1) You have 10. 0 moles H 2 and 1. 00 mol O 2. How much H 2 O can you make? 2) You combust 10. 2 mol propane in 7. 80 mol O 2. How much CO 2 can you produce?

3) If 2. 3 mol carbon disulfide reacts with 5. 4 mol oxygen to form carbon dioxide and sulfur dioxide, what mass of sulfur dioxide is formed? 4) 5. 50 g silicon dioxide reacts with 4. 71 g Carbon to from silicon carbide and carbon monoxide. What mass of carbon monoxide is formed?

Problems 1) From the previous question, theoretical yield of CO is 5. 13 g. If you obtained 4. 32 g CO, what was your % yield? 2) You react 4. 41 mol carbon monoxide with 8. 39 mol hydrogen gas to get 122 g methanol. What is your percent yield?