Chapter 5 Chemical Reactions 5 3 OxidationReduction Reactions




- Slides: 23

Chapter 5 Chemical Reactions 5. 3 Oxidation-Reduction Reactions 1

Oxidation and Reduction An oxidation-reduction reaction • provides us with energy from food. • provides electrical energy in batteries. • occurs when iron rusts. 4 Fe(s) + 3 O 2(g) 2 Fe 2 O 3(s) 2

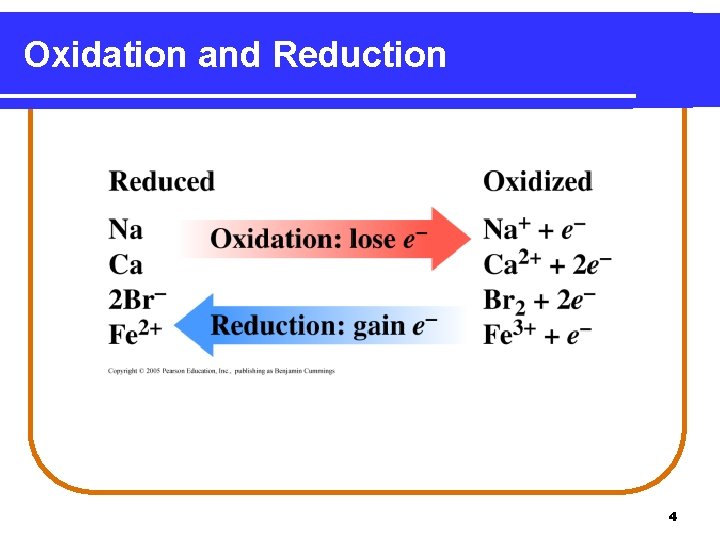
Electron Loss and Gain An oxidation-reduction reaction • transfers electrons from one reactant to another. • loses electrons in oxidation. (LEO) or (OIL) Zn(s) Zn 2+(aq) + 2 e- (loss of electrons) • gains electrons in reduction. (GER) or (RIG) Cu 2+(aq) + 2 e. Cu(s) (gain of electrons) 3

Oxidation and Reduction 4

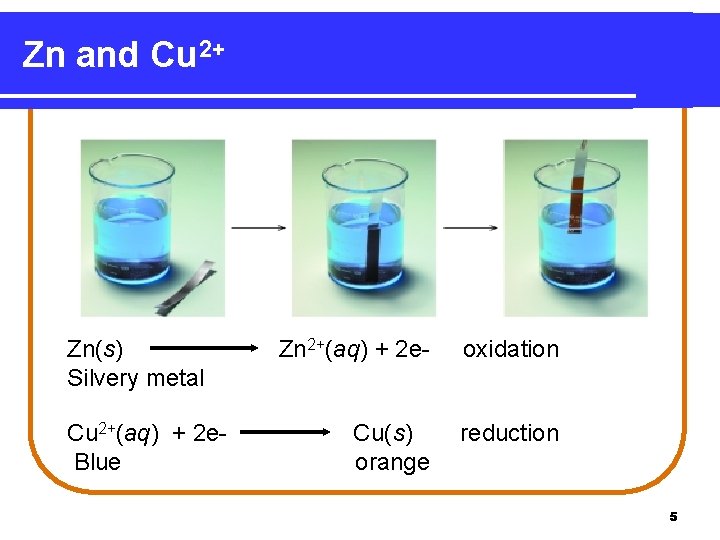
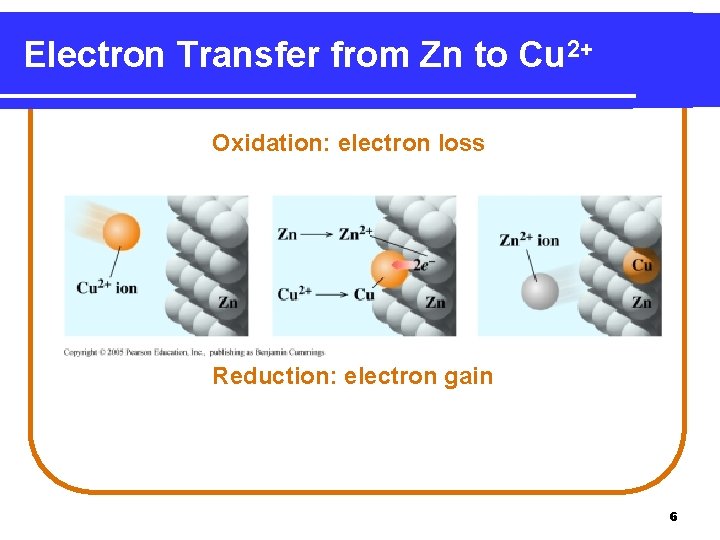
Zn and Cu 2+ Zn(s) Silvery metal Cu 2+(aq) + 2 e. Blue Zn 2+(aq) + 2 e- oxidation Cu(s) orange reduction 5

Electron Transfer from Zn to Cu 2+ Oxidation: electron loss Reduction: electron gain 6

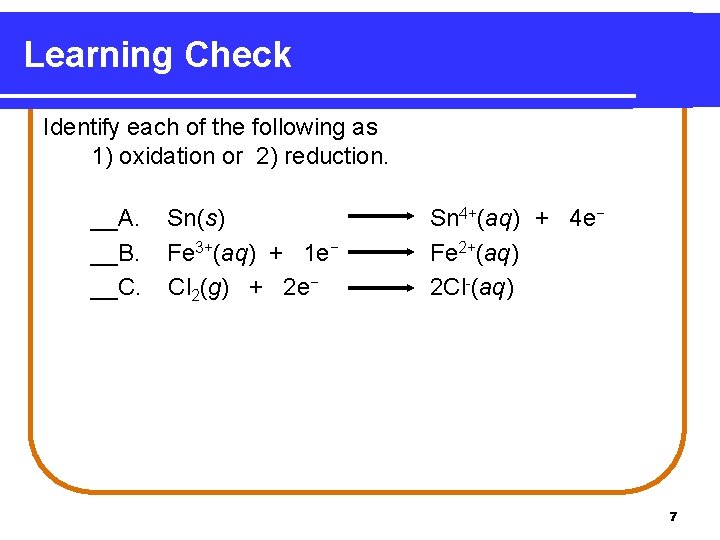
Learning Check Identify each of the following as 1) oxidation or 2) reduction. __A. __B. __C. Sn(s) Fe 3+(aq) + 1 e− Cl 2(g) + 2 e− Sn 4+(aq) + 4 e− Fe 2+(aq) 2 Cl-(aq) 7

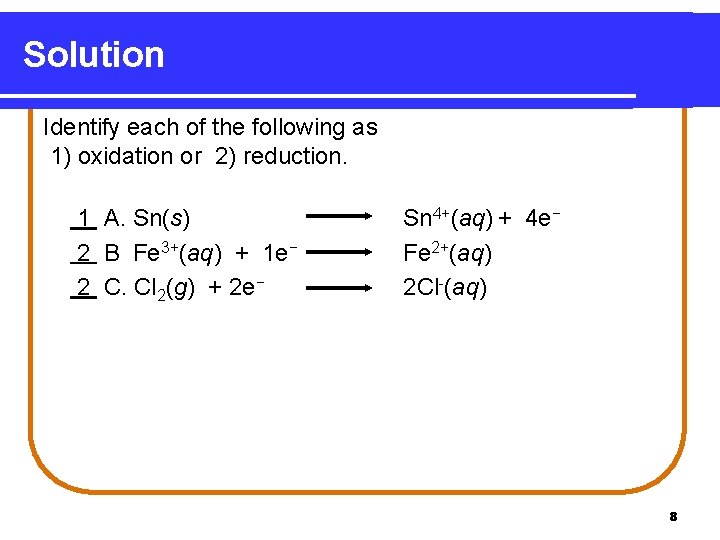
Solution Identify each of the following as 1) oxidation or 2) reduction. 1 A. Sn(s) 2 B Fe 3+(aq) + 1 e− 2 C. Cl 2(g) + 2 e− Sn 4+(aq) + 4 e− Fe 2+(aq) 2 Cl-(aq) 8

Writing Oxidation and Reduction Reactions Write the separate oxidation and reduction reactions for the following equation. 2 Cs(s) + F 2(g) 2 Cs. F(s) A cesium atom loses an electron to form cesium ion. Cs(s) Cs+(s) + 1 e− oxidation Fluorine atoms gain electrons to form fluoride ions. F 2(s) + 2 e- 2 F−(s) reduction 9

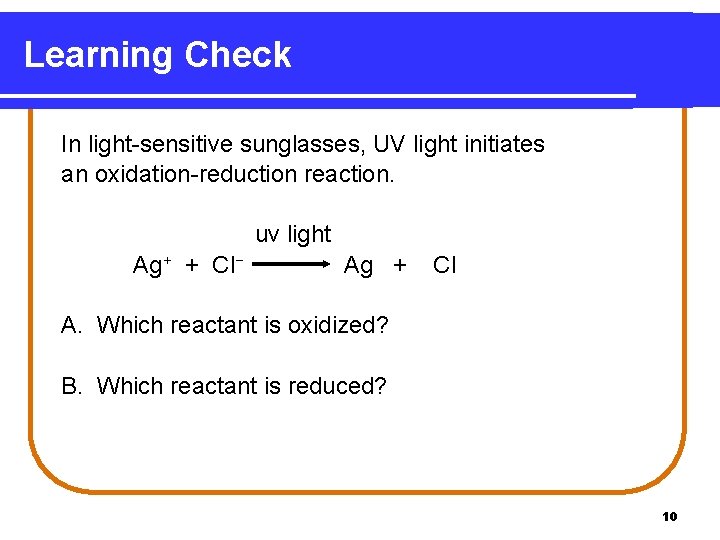
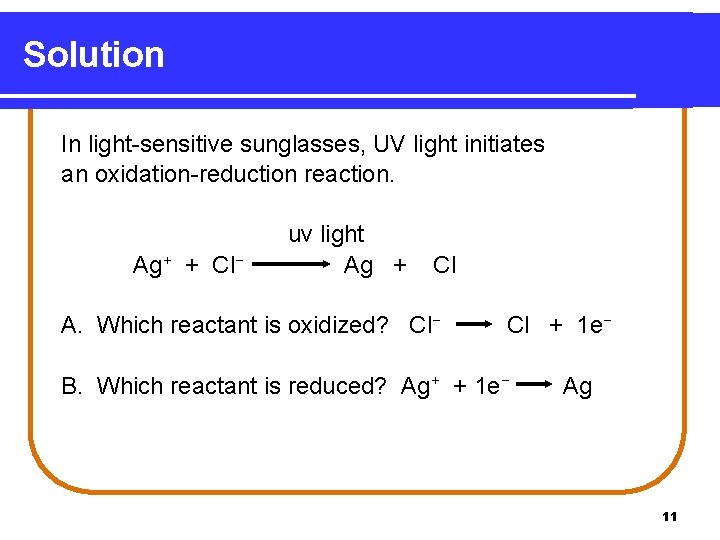
Learning Check In light-sensitive sunglasses, UV light initiates an oxidation-reduction reaction. uv light Ag+ + Cl− Ag + Cl A. Which reactant is oxidized? B. Which reactant is reduced? 10

Solution In light-sensitive sunglasses, UV light initiates an oxidation-reduction reaction. Ag+ + Cl− uv light Ag + Cl A. Which reactant is oxidized? Cl− Cl + 1 e− B. Which reactant is reduced? Ag+ + 1 e− Ag 11

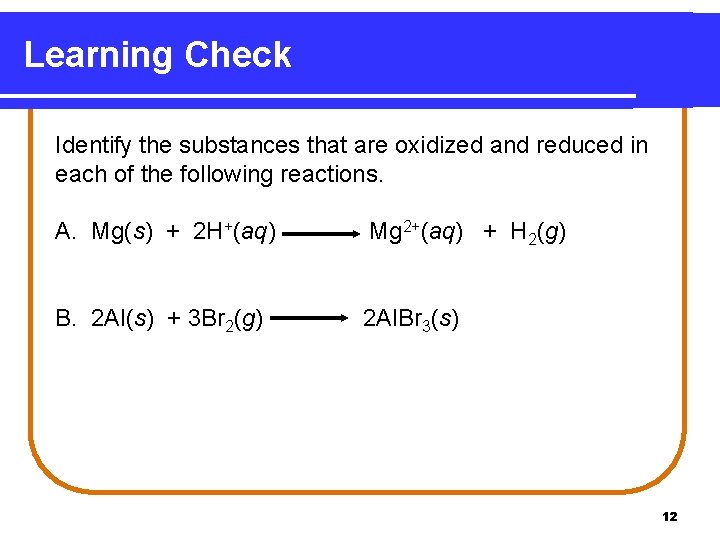
Learning Check Identify the substances that are oxidized and reduced in each of the following reactions. A. Mg(s) + 2 H+(aq) Mg 2+(aq) + H 2(g) B. 2 Al(s) + 3 Br 2(g) 2 Al. Br 3(s) 12

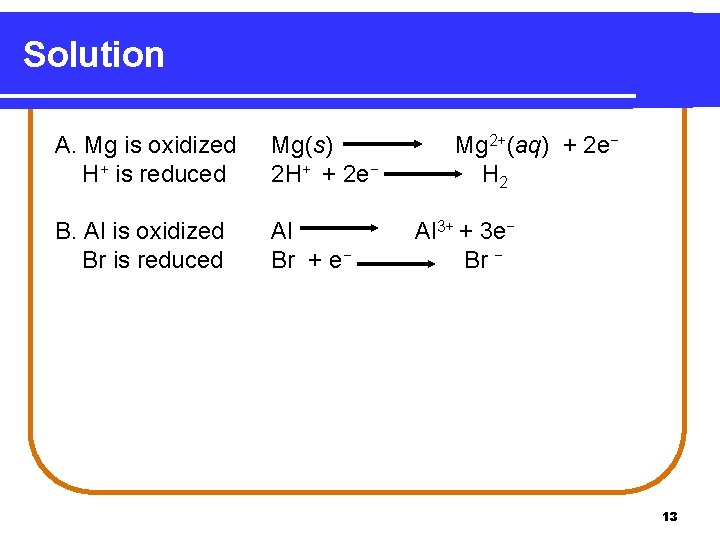
Solution A. Mg is oxidized H+ is reduced Mg(s) 2 H+ + 2 e− B. Al is oxidized Br is reduced Al Br + e− Mg 2+(aq) + 2 e− H 2 Al 3+ + 3 e− Br − 13

Common uses of the terms oxidization and reduction Term Meaning Oxidation To combine with oxygen To lose hydrogen To lose electrons To increase in oxidation number Reduction To lose oxygen To combine with hydrogen To gain electrons To decrease in oxidation number 14

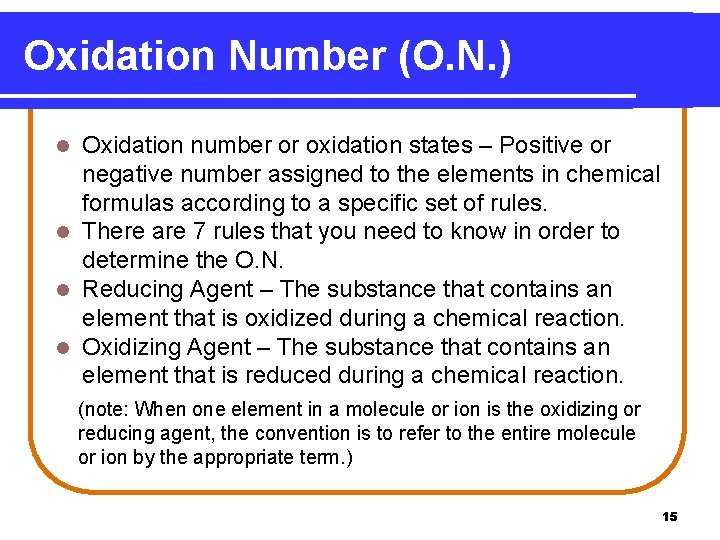
Oxidation Number (O. N. ) Oxidation number or oxidation states – Positive or negative number assigned to the elements in chemical formulas according to a specific set of rules. l There are 7 rules that you need to know in order to determine the O. N. l Reducing Agent – The substance that contains an element that is oxidized during a chemical reaction. l Oxidizing Agent – The substance that contains an element that is reduced during a chemical reaction. l (note: When one element in a molecule or ion is the oxidizing or reducing agent, the convention is to refer to the entire molecule or ion by the appropriate term. ) 15

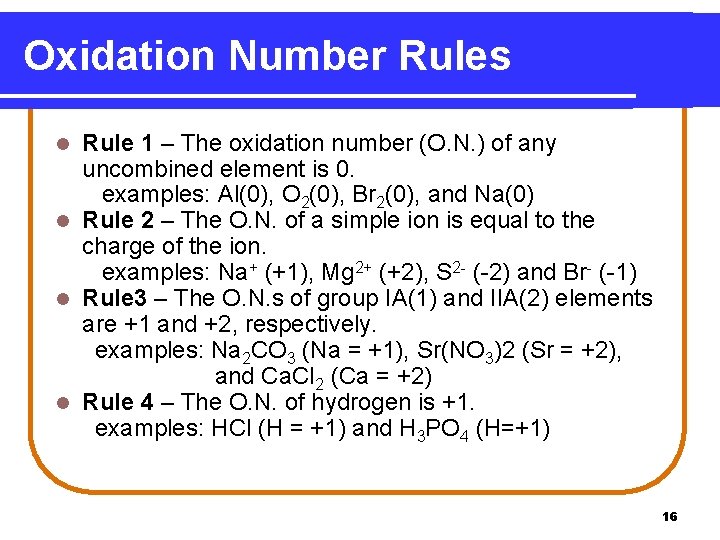
Oxidation Number Rules Rule 1 – The oxidation number (O. N. ) of any uncombined element is 0. examples: Al(0), O 2(0), Br 2(0), and Na(0) l Rule 2 – The O. N. of a simple ion is equal to the charge of the ion. examples: Na+ (+1), Mg 2+ (+2), S 2 - (-2) and Br- (-1) l Rule 3 – The O. N. s of group IA(1) and IIA(2) elements are +1 and +2, respectively. examples: Na 2 CO 3 (Na = +1), Sr(NO 3)2 (Sr = +2), and Ca. Cl 2 (Ca = +2) l Rule 4 – The O. N. of hydrogen is +1. examples: HCl (H = +1) and H 3 PO 4 (H=+1) l 16

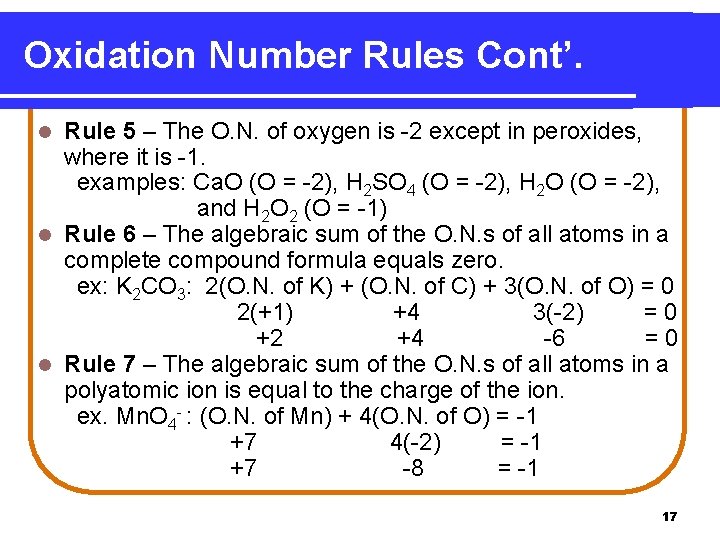
Oxidation Number Rules Cont’. Rule 5 – The O. N. of oxygen is -2 except in peroxides, where it is -1. examples: Ca. O (O = -2), H 2 SO 4 (O = -2), H 2 O (O = -2), and H 2 O 2 (O = -1) l Rule 6 – The algebraic sum of the O. N. s of all atoms in a complete compound formula equals zero. ex: K 2 CO 3: 2(O. N. of K) + (O. N. of C) + 3(O. N. of O) = 0 2(+1) +4 3(-2) =0 +2 +4 -6 =0 l Rule 7 – The algebraic sum of the O. N. s of all atoms in a polyatomic ion is equal to the charge of the ion. ex. Mn. O 4 - : (O. N. of Mn) + 4(O. N. of O) = -1 +7 4(-2) = -1 +7 -8 = -1 l 17

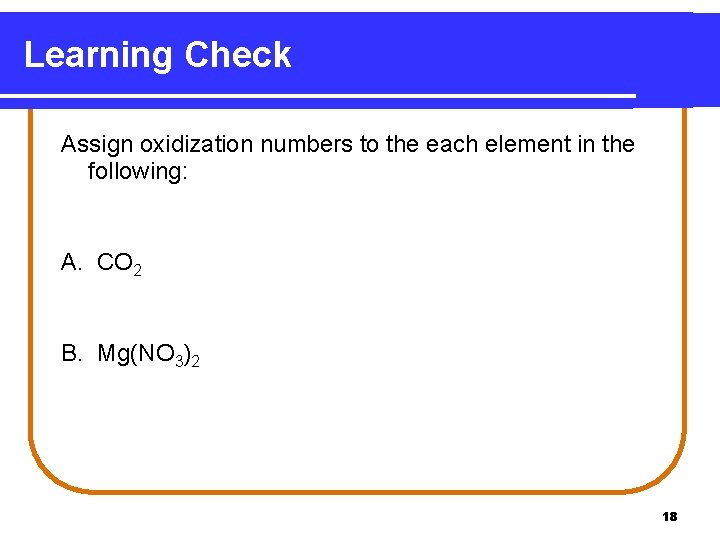
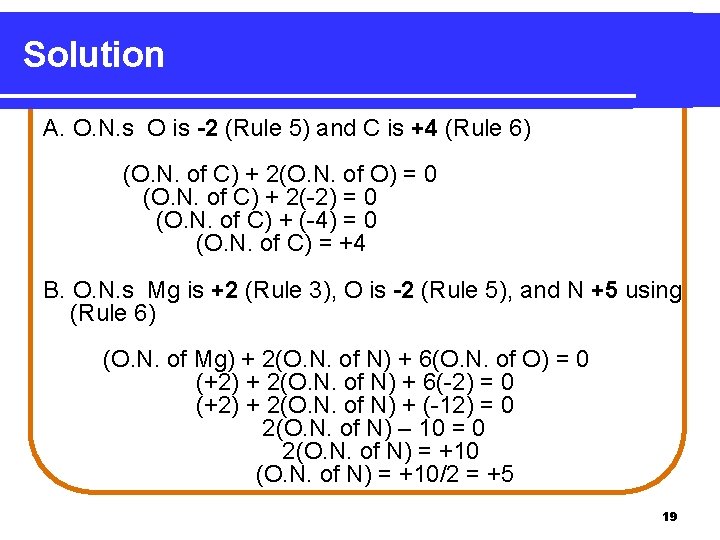
Learning Check Assign oxidization numbers to the each element in the following: A. CO 2 B. Mg(NO 3)2 18

Solution A. O. N. s O is -2 (Rule 5) and C is +4 (Rule 6) (O. N. of C) + 2(O. N. of O) = 0 (O. N. of C) + 2(-2) = 0 (O. N. of C) + (-4) = 0 (O. N. of C) = +4 B. O. N. s Mg is +2 (Rule 3), O is -2 (Rule 5), and N +5 using (Rule 6) (O. N. of Mg) + 2(O. N. of N) + 6(O. N. of O) = 0 (+2) + 2(O. N. of N) + 6(-2) = 0 (+2) + 2(O. N. of N) + (-12) = 0 2(O. N. of N) – 10 = 0 2(O. N. of N) = +10/2 = +5 19

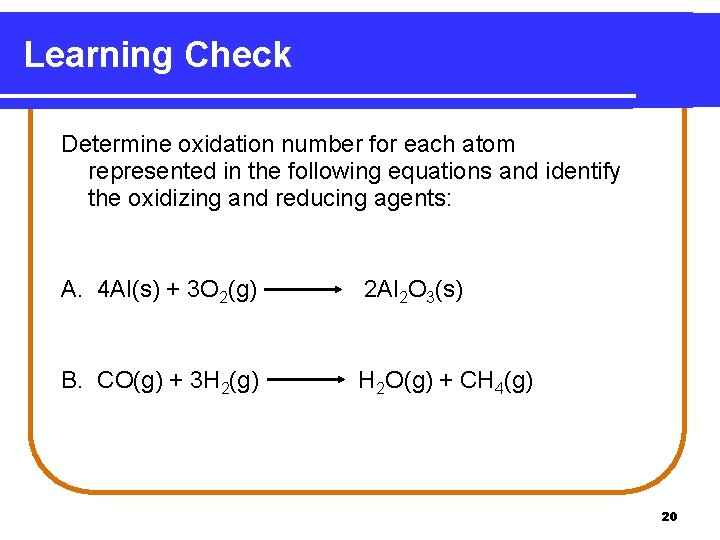
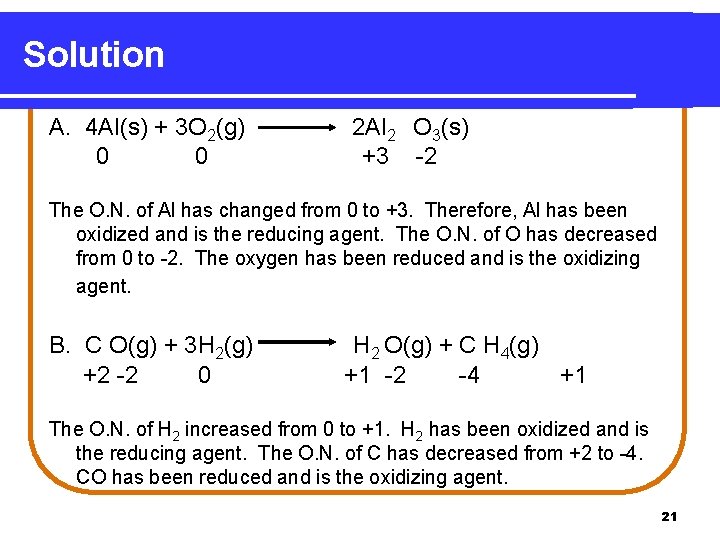
Learning Check Determine oxidation number for each atom represented in the following equations and identify the oxidizing and reducing agents: A. 4 Al(s) + 3 O 2(g) 2 Al 2 O 3(s) B. CO(g) + 3 H 2(g) H 2 O(g) + CH 4(g) 20

Solution A. 4 Al(s) + 3 O 2(g) 0 0 2 Al 2 O 3(s) +3 -2 The O. N. of Al has changed from 0 to +3. Therefore, Al has been oxidized and is the reducing agent. The O. N. of O has decreased from 0 to -2. The oxygen has been reduced and is the oxidizing agent. B. C O(g) + 3 H 2(g) +2 -2 0 H 2 O(g) + C H 4(g) +1 -2 -4 +1 The O. N. of H 2 increased from 0 to +1. H 2 has been oxidized and is the reducing agent. The O. N. of C has decreased from +2 to -4. CO has been reduced and is the oxidizing agent. 21

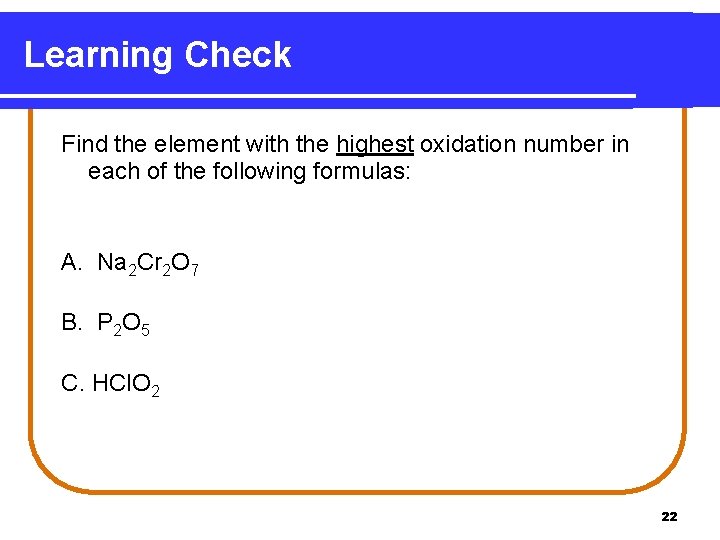
Learning Check Find the element with the highest oxidation number in each of the following formulas: A. Na 2 Cr 2 O 7 B. P 2 O 5 C. HCl. O 2 22

Solution A. Cr (+6) B. P (+5) C. Cl (+3) 23