Chapter 5 Chemical Reactions 5 1 Chemical Equations





- Slides: 22

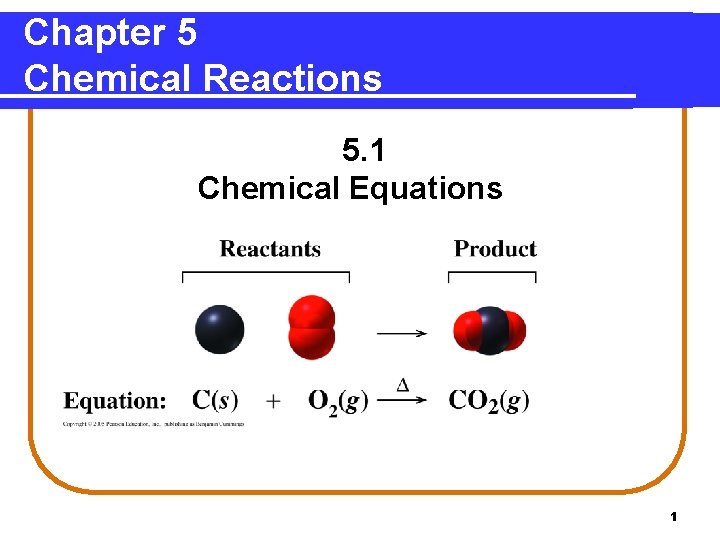
Chapter 5 Chemical Reactions 5. 1 Chemical Equations 1

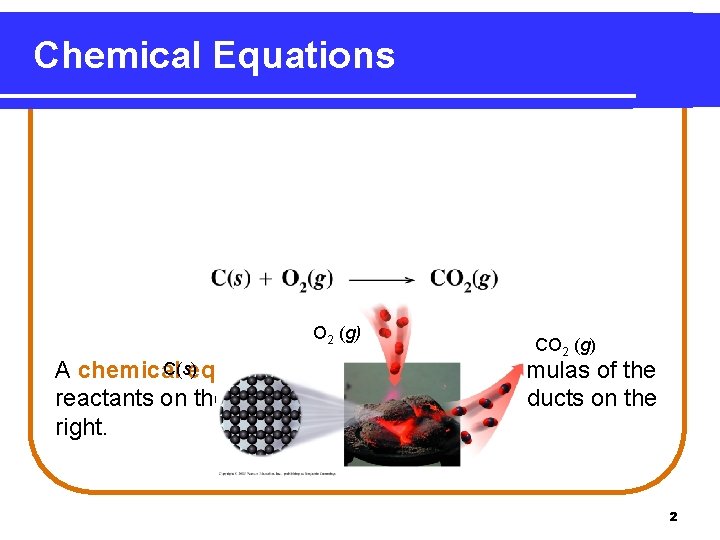
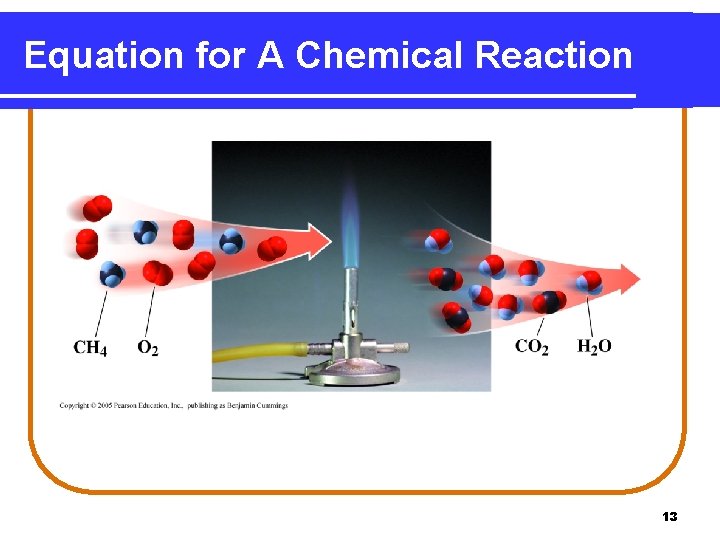
Chemical Equations O 2 (g) C(s) A chemical equation gives the chemical formulas of the reactants on the left of the arrow and the products on the right. Reactants Product 2

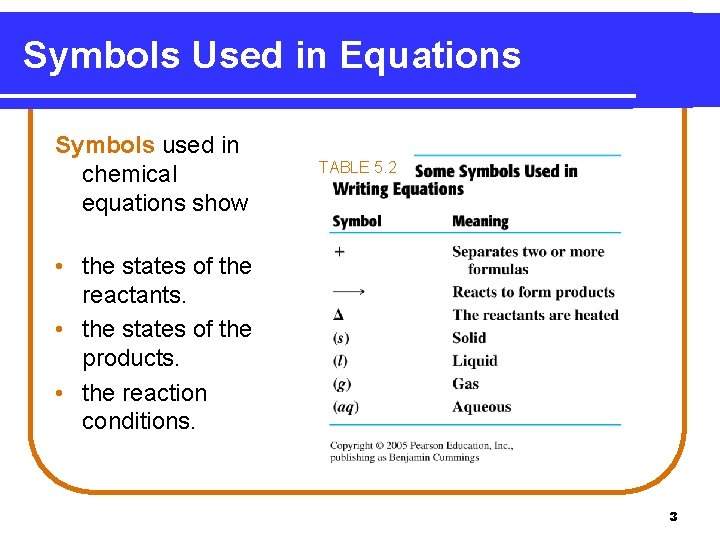
Symbols Used in Equations Symbols used in chemical equations show TABLE 5. 2 • the states of the reactants. • the states of the products. • the reaction conditions. 3

Chemical Equations are Balanced In a balanced chemical reaction • atoms are not gained or lost. • the number of reactant atoms is equal to the number of product atoms. 4

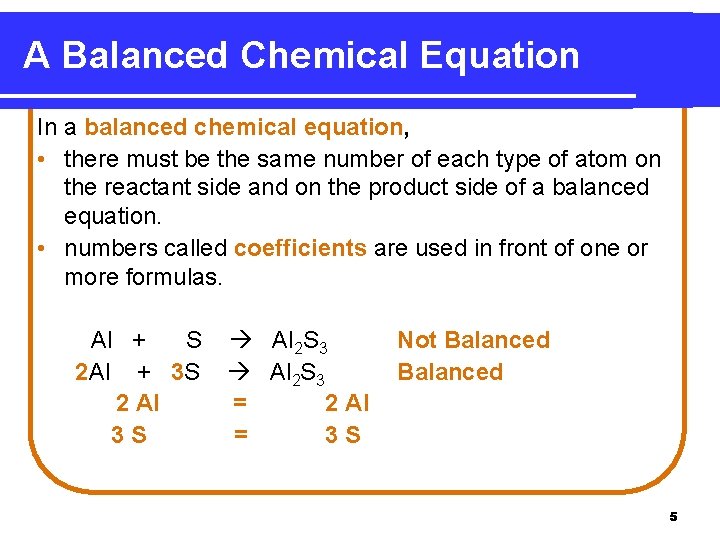
A Balanced Chemical Equation In a balanced chemical equation, • there must be the same number of each type of atom on the reactant side and on the product side of a balanced equation. • numbers called coefficients are used in front of one or more formulas. Al + S 2 Al + 3 S 2 Al 3 S Al 2 S 3 = 2 Al = 3 S Not Balanced 5

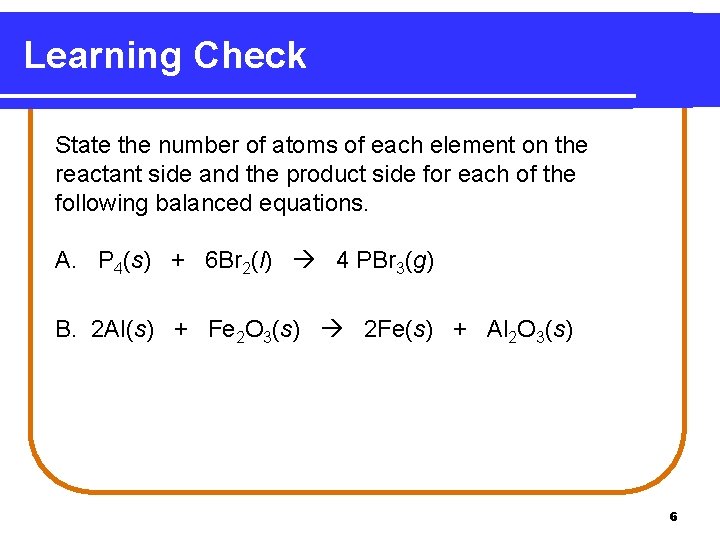
Learning Check State the number of atoms of each element on the reactant side and the product side for each of the following balanced equations. A. P 4(s) + 6 Br 2(l) 4 PBr 3(g) B. 2 Al(s) + Fe 2 O 3(s) 2 Fe(s) + Al 2 O 3(s) 6

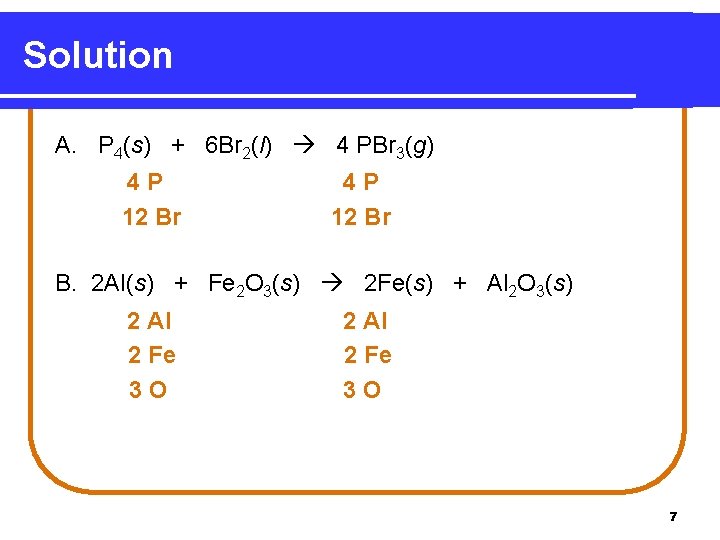
Solution A. P 4(s) + 6 Br 2(l) 4 PBr 3(g) 4 P 12 Br B. 2 Al(s) + Fe 2 O 3(s) 2 Fe(s) + Al 2 O 3(s) 2 Al 2 Fe 3 O 7

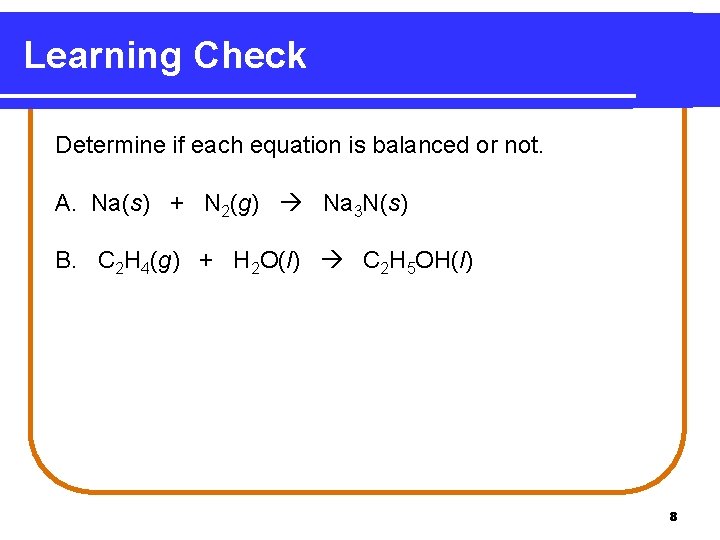
Learning Check Determine if each equation is balanced or not. A. Na(s) + N 2(g) Na 3 N(s) B. C 2 H 4(g) + H 2 O(l) C 2 H 5 OH(l) 8

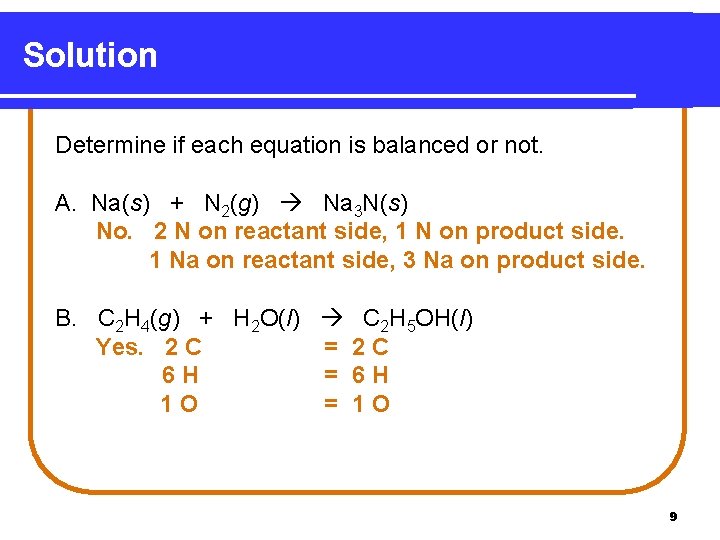
Solution Determine if each equation is balanced or not. A. Na(s) + N 2(g) Na 3 N(s) No. 2 N on reactant side, 1 N on product side. 1 Na on reactant side, 3 Na on product side. B. C 2 H 4(g) + H 2 O(l) C 2 H 5 OH(l) Yes. 2 C = 2 C 6 H = 6 H 1 O = 1 O 9

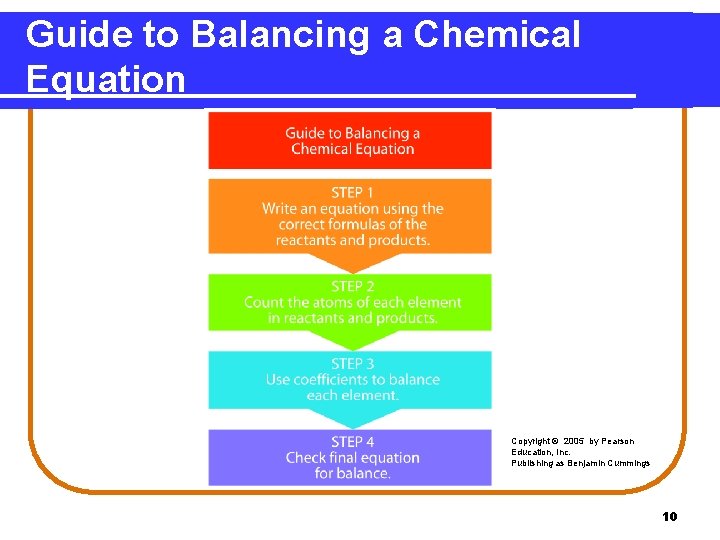
Guide to Balancing a Chemical Equation Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 10

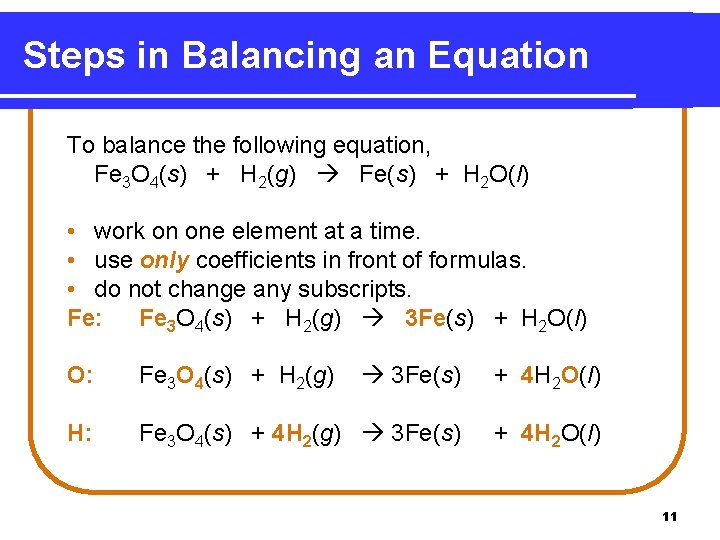
Steps in Balancing an Equation To balance the following equation, Fe 3 O 4(s) + H 2(g) Fe(s) + H 2 O(l) • work on one element at a time. • use only coefficients in front of formulas. • do not change any subscripts. Fe: Fe 3 O 4(s) + H 2(g) 3 Fe(s) + H 2 O(l) O: Fe 3 O 4(s) + H 2(g) 3 Fe(s) + 4 H 2 O(l) H: Fe 3 O 4(s) + 4 H 2(g) 3 Fe(s) + 4 H 2 O(l) 11

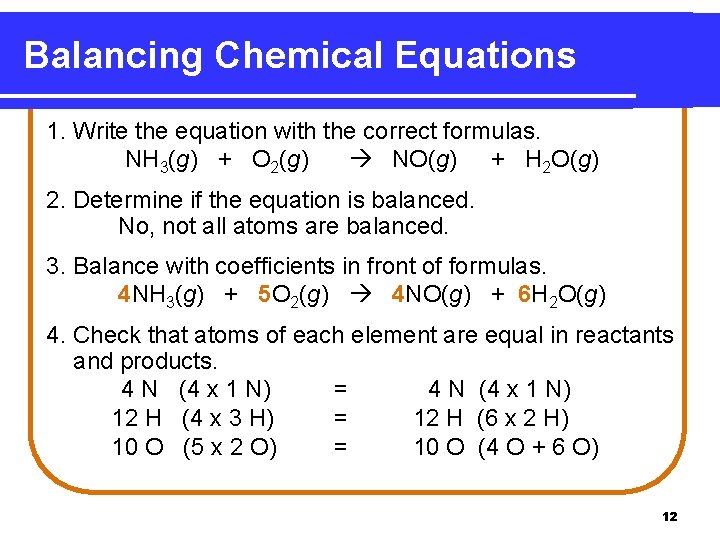
Balancing Chemical Equations 1. Write the equation with the correct formulas. NH 3(g) + O 2(g) NO(g) + H 2 O(g) 2. Determine if the equation is balanced. No, not all atoms are balanced. 3. Balance with coefficients in front of formulas. 4 NH 3(g) + 5 O 2(g) 4 NO(g) + 6 H 2 O(g) 4. Check that atoms of each element are equal in reactants and products. 4 N (4 x 1 N) = 4 N (4 x 1 N) 12 H (4 x 3 H) = 12 H (6 x 2 H) 10 O (5 x 2 O) = 10 O (4 O + 6 O) 12

Equation for A Chemical Reaction 13

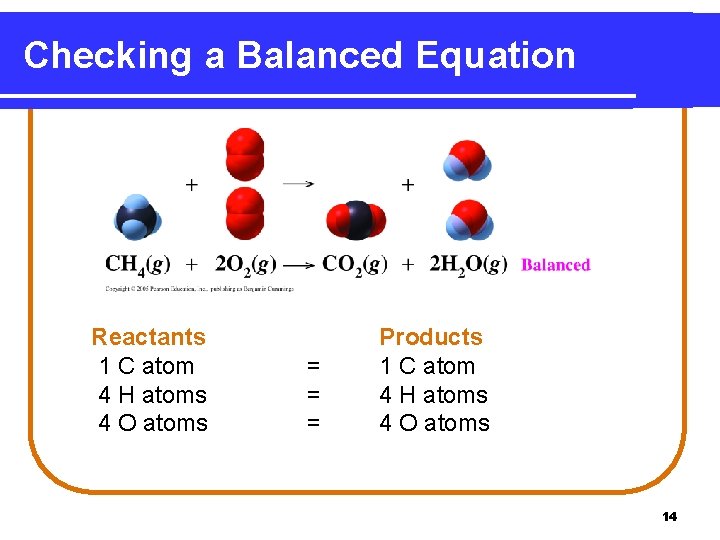
Checking a Balanced Equation Reactants 1 C atom 4 H atoms 4 O atoms = = = Products 1 C atom 4 H atoms 4 O atoms 14

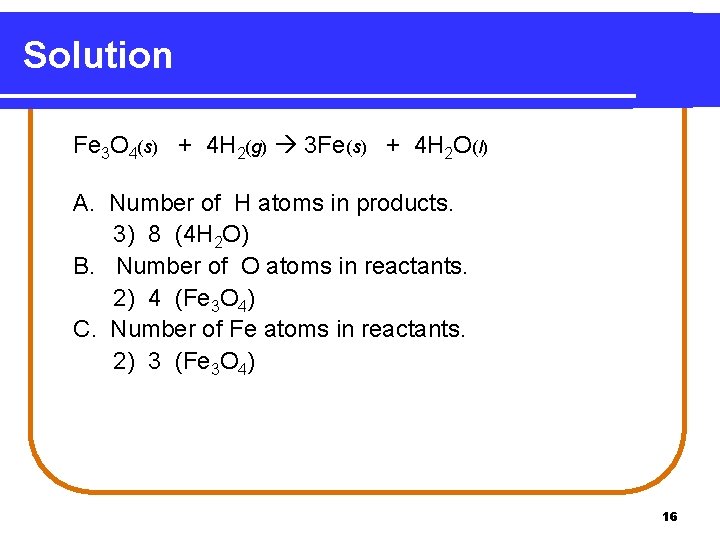
Learning Check the balance of atoms in the following. Fe 3 O 4(s) + 4 H 2(g) 3 Fe(s) + 4 H 2 O(l) A. Number of H atoms in products. 1) 2 2) 4 3) 8 B. Number of O atoms in reactants. 1) 2 2) 4 3) 8 C. Number of Fe atoms in reactants. 1) 1 2) 3 3) 4 15

Solution Fe 3 O 4(s) + 4 H 2(g) 3 Fe(s) + 4 H 2 O(l) A. Number of H atoms in products. 3) 8 (4 H 2 O) B. Number of O atoms in reactants. 2) 4 (Fe 3 O 4) C. Number of Fe atoms in reactants. 2) 3 (Fe 3 O 4) 16

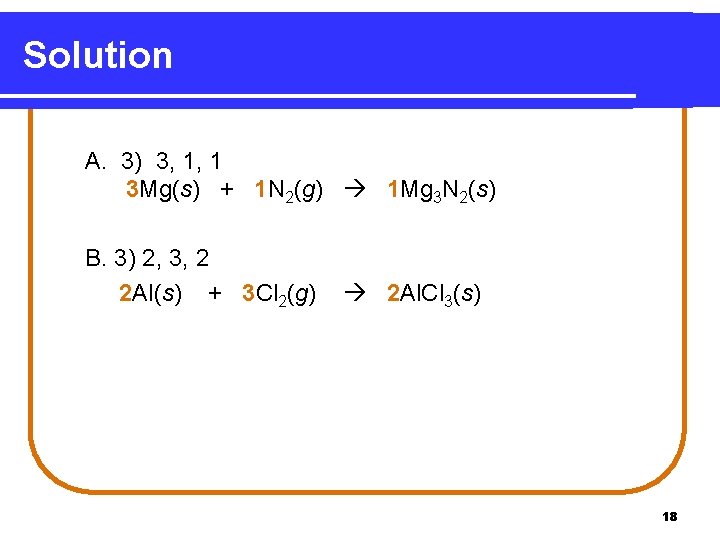
Learning Check Balance each equation and list the coefficients in the balanced equation going from reactants to products: A. __Mg(s) 1) 1, 3, 2 B. __Al(s) 1) 3, 3, 2 + __N 2(g) __Mg 3 N 2(s) 2) 3, 1, 2 3) 3, 1, 1 + __Cl 2(g) 2) 1, 3, 1 __Al. Cl 3(s) 3) 2, 3, 2 17

Solution A. 3) 3, 1, 1 3 Mg(s) + 1 N 2(g) 1 Mg 3 N 2(s) B. 3) 2, 3, 2 2 Al(s) + 3 Cl 2(g) 2 Al. Cl 3(s) 18

Equations with Polyatomic Ions 19

Balancing with Polyatomic Ions Mg. Cl 2(aq) + Na 3 PO 4(aq) Na. Cl(aq) + Mg 3(PO 4)2(s) Balance PO 43 - as a unit Mg. Cl 2(aq) + 2 Na 3 PO 4(aq) Na. Cl(aq) + Mg 3(PO 4)2(s) 2 PO 43= 2 PO 43 - Balance Mg and Cl 3 Mg. Cl 2(aq) + 2 Na 3 PO 4(aq) 6 Na. Cl(aq) + Mg 3(PO 4)2(s) 3 Mg 2+ = 3 Mg 2+ 6 Na+ = 6 Na+ 6 Cl= 6 Cl 20

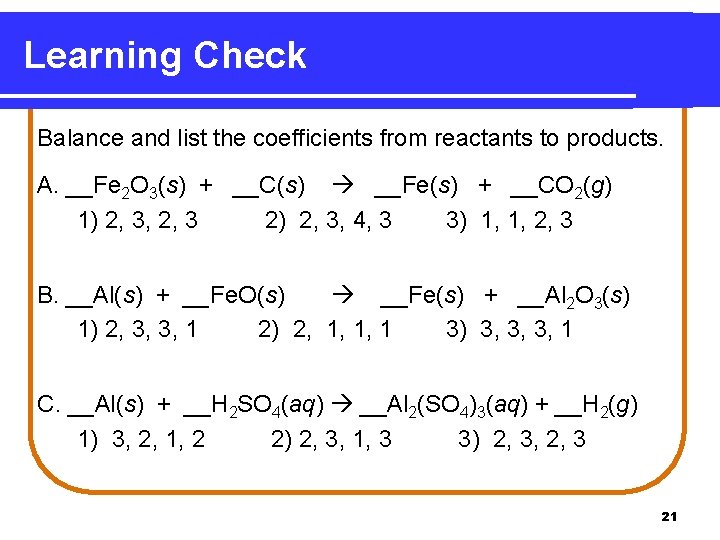
Learning Check Balance and list the coefficients from reactants to products. A. __Fe 2 O 3(s) + __C(s) __Fe(s) + __CO 2(g) 1) 2, 3, 2, 3 2) 2, 3, 4, 3 3) 1, 1, 2, 3 B. __Al(s) + __Fe. O(s) __Fe(s) + __Al 2 O 3(s) 1) 2, 3, 3, 1 2) 2, 1, 1, 1 3) 3, 3, 3, 1 C. __Al(s) + __H 2 SO 4(aq) __Al 2(SO 4)3(aq) + __H 2(g) 1) 3, 2, 1, 2 2) 2, 3, 1, 3 3) 2, 3, 2, 3 21

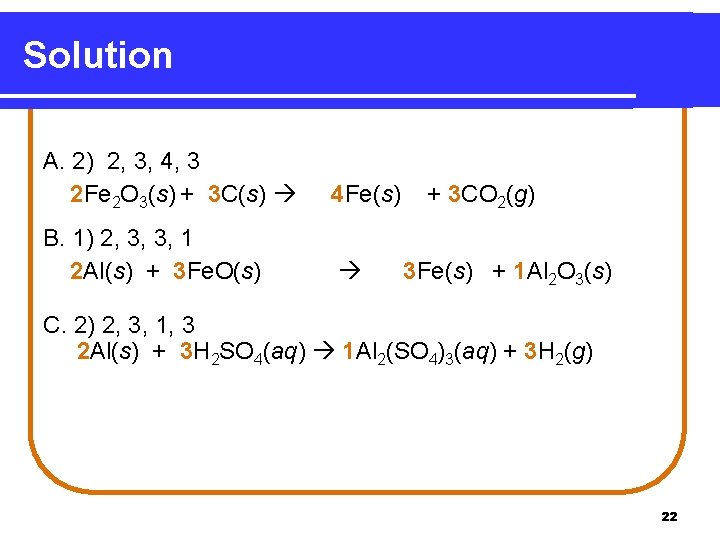
Solution A. 2) 2, 3, 4, 3 2 Fe 2 O 3(s) + 3 C(s) 4 Fe(s) B. 1) 2, 3, 3, 1 2 Al(s) + 3 Fe. O(s) + 3 CO 2(g) 3 Fe(s) + 1 Al 2 O 3(s) C. 2) 2, 3, 1, 3 2 Al(s) + 3 H 2 SO 4(aq) 1 Al 2(SO 4)3(aq) + 3 H 2(g) 22
 Are kc and kp equal
Are kc and kp equal Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Chapter 8 review chemical equations and reactions section 2
Chapter 8 review chemical equations and reactions section 2 Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Chapter 8 review chemical equations and reactions
Chapter 8 review chemical equations and reactions Chapter 19 chemical reactions answer key
Chapter 19 chemical reactions answer key Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Toxic reactions chemical equations worksheet answers
Toxic reactions chemical equations worksheet answers Hcl and sodium hydrogen carbonate
Hcl and sodium hydrogen carbonate Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Toxic reactions chemical equations
Toxic reactions chemical equations Chapter 10 chapter assessment chemical reactions answers
Chapter 10 chapter assessment chemical reactions answers Chapter 9 chapter assessment chemical reactions
Chapter 9 chapter assessment chemical reactions Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Basic redox reactions
Basic redox reactions Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Translate word equations to chemical equations
Translate word equations to chemical equations Chapter 9 chemical reactions
Chapter 9 chemical reactions Chemical reactions chapter 9 study guide
Chemical reactions chapter 9 study guide Chapter 11 chemical reactions answer key
Chapter 11 chemical reactions answer key Chapter 11 chemical reactions practice problems
Chapter 11 chemical reactions practice problems Examples of double replacement reactions
Examples of double replacement reactions