Chapter 5 Bonding in polyatomic molecules TOPICS Hybridization

Chapter 5 Bonding in polyatomic molecules TOPICS §Hybridization of atomic orbitals §Molecular orbital theory: ligand group orbitals §Delocalized bonding §Partial molecular orbital treatments

5. 1 Introduction Polyatomic species: contains three or more atoms Three approaches to bonding in diatomic molecules 1. Lewis structures 2. Valence bond theory 3. Molecular orbital theory

5. 2 Valence bond theory: hybridization of atomic orbitals Hybrid orbitals are generated by mixing the characters of atomic orbitals. A set of hybrid orbitals provides a bonding picture for a molecule in terms of localized s - bonds. sp Hybridization: a scheme for linear species. The notation sp means that one s atomic orbital and one p atomic orbital mix to form a set of two hybrid orbitals with different directional properties.

If we begin with n atomic orbitals, we must end up with n orbitals after hybridization. Effectively, we are representing the valence state of Be in a linear molecule as consisting of two degenerate sp hybrids, each containing one electron; this is represented by the notation (sp)2.

sp 2 Hybridization: a scheme for trigonal planar species The notation sp 2 means that one s and two p atomic orbitals mix to form a set of three hybrid orbitals with different directional properties.

The probability of finding the electron somewhere in space is taken to be 1.

Fig. 5. 5 The bonding in trigonal planar BH 3 can be conveniently described in terms of the interactions between a set of sp 2 hybrid orbitals centred on the B atom and three H 1 s atomic orbitals. Three pairs of electrons are available (three electrons from B and one from each H) to give three 2 c-2 e s -bonds.

sp 3 Hybridization: a scheme for tetrahedral and related species The notation sp 3 means that one s and three p atomic orbitals mix to form a set of four hybrid orbitals with different directional properties. Fig. 5. 6 (a) The directions of the orbitals that make up a set of four sp 3 hybrid orbitals correspond to a tetrahedral array. (b) The relationship between a tetrahedron and a cube; in CH 4, the four H atoms occupy alternate corners of a cube, and the cube is easily related to a Cartesian axis set.

Worked example 5. 1 Hybridization scheme for the nitrogen atom in NH 3 Use VSEPR theory to account for the structure of NH 3, and suggest an appropriate hybridization scheme for the N atom.

Other hybridization schemes sp 3 d hybrid orbitals: one s, three p, and one d atomic orbitals mix to form a set of five orbitals with different directional properties


5. 3 Valence bond theory: multiple bonding in polyatomic molecules C 2 H 4 C [He]2 s 22 p 2 H 1 s 1 The p-component of the overall carbon–carbon bond is weaker than the s-component and hence a C=C double bond, though stronger than a C C single bond, is not twice as strong; the C C bond enthalpy terms in C 2 H 4 and C 2 H 6 are 598 and 346 k. J mol 1 respectively. Fig. 5. 8 (a) Ethene is a planar molecule with H C H and C C H bond angles close to 120 o. (b) An sp 2 hybridization scheme is appropriate to describe the s-bonding framework. (c) This leaves a 2 p atomic orbital on each C atom; overlap between them gives a C C p-interaction.
![HCN C [He]2 s 22 p 2 N [He]2 s 22 p 3 H HCN C [He]2 s 22 p 2 N [He]2 s 22 p 3 H](http://slidetodoc.com/presentation_image/4243b49e2514ee52a06d029dffc8f1b0/image-13.jpg)
HCN C [He]2 s 22 p 2 N [He]2 s 22 p 3 H 1 s 1 Fig. 5. 9 (a) The linear structure of HCN; colour code: C, grey; N, blue; H, white. (b) An sp hybridization scheme for C and N can be used to describe the s -bonding in HCN. (c) The p-character in the C N bond arises from 2 p– 2 p overlap.
![BF 3 B [He]2 s 22 p 1 F [He]2 s 22 p 5 BF 3 B [He]2 s 22 p 1 F [He]2 s 22 p 5](http://slidetodoc.com/presentation_image/4243b49e2514ee52a06d029dffc8f1b0/image-14.jpg)
BF 3 B [He]2 s 22 p 1 F [He]2 s 22 p 5 Fig. 5. 10 (a) BF 3 possesses a trigonal planar structure. (b) 2 p– 2 p overlap between B and F leads to the formation of a p -interaction. (c) Boron–fluorine double bond character is also deduced by considering the resonance structures for BF 3; only those forms that contribute significantly are shown.
![Worked example 5. 2 Valence bond treatment of the bonding in [NO 3] (a) Worked example 5. 2 Valence bond treatment of the bonding in [NO 3] (a)](http://slidetodoc.com/presentation_image/4243b49e2514ee52a06d029dffc8f1b0/image-15.jpg)
Worked example 5. 2 Valence bond treatment of the bonding in [NO 3] (a) The [NO 3] ion has D 3 h symmetry. What does this tell you about its structure? (b) Draw a set of resonance structures (focusing only on those that contribute significantly) for the nitrate ion. (c) Use an appropriate hybridization scheme to describe the bonding in [NO 3] .

(c) Using a hybridization scheme, we should end up with a bonding picture that corresponds to that depicted by the resonance structures. N O s–bonds is beween sp 2 hybrid Orbital of N and sp 2 hybrid orbital of O. The next step is to consider multiple bonding character. Each N and O atom has an unused 2 p atomic orbital lying perpendicular to the plane of the molecule. Overlap between the 2 p atomic orbital on nitrogen with one of those on an oxygen atom gives rise to one localized p -bond.

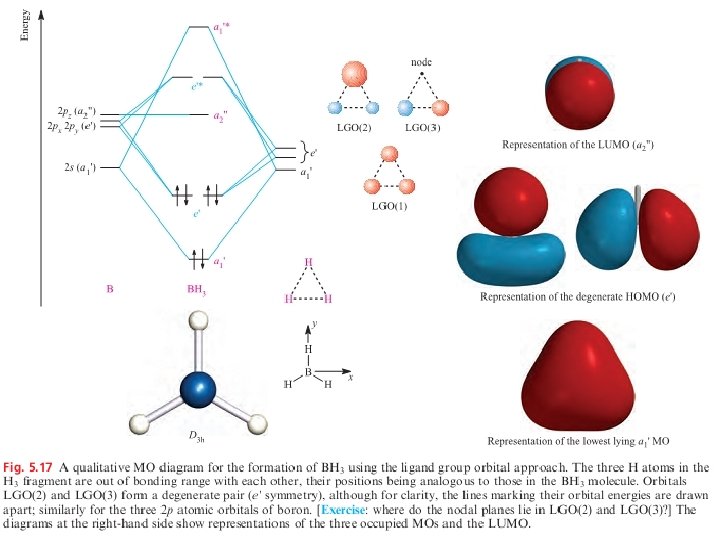
Molecular orbital diagrams: ligand group orbital approach in triatomic molecules In order to overcome the difficulty of drawing MO diagram for four sets of orbitals or more, it is common to resolve the MO description of a poly atomic molecule into a three component problem , a method known as the ligand group orbital (LGO) approach. Consider the two 1 s a tomic orbitals of the two H atoms. Each 1 s atomic orbital has two possible phases and, when the two 1 s orbitals are taken as a group , there are two possible phase combinations. The number of ligand group orbitals formed = the number of atomic orbitals used.

In constructing an MO diagram for XH 2 (Figure 5. 11), we consider the interactions of the valence atomic orbitals of X with the ligand group orbitals of the H ----- H fragment. Ligand group orbital LGO(1) has the correct symmetry to interact with the 2 s atomic orbital of X, giving an MO with H- X- H s-bonding character. The symmetry of LGO(2) is matched to that of the 2 pz atomic orbital of X. An important result of the MO treatment of the bonding in XH 2 is that the s -bonding character in orbitals 1 and 2 is spread over all three atoms, indicating that the bonding character is delocalized over the H-X -H framework. Delocalized bonding is a general result within MO theory.


A bent triatomic: H 2 O

Mulliken Symbol Notation 1)- A or B: 1 -dimensional representations E : 2 -dimensional representations T : 3 -dimensional representations 2)- A = symmetric with respect to rotation by the Cn axis B = anti-symmetric w/respect to rotation by Cn axis Symmetric = + (positive) character Anti-symmetric = (negative) character Subscripts 1 and 2 associated with A and B symbols indicate whether a C 2 axis to the principle axis produces a symmetric (1) or anti-symmetric (2) result. Although the symmetry labels in the character tables are upper case (e. g. A 1, E, T 2 g) The corresponding symmetry labels for orbitals are lower case (e. g. a 1, e, t 2 g).

To illustrate its use, let us consider the 2 s atomic orbital of the O atom in water : Apply each symmetry operation of the C 2 v point group in turn. Applying the E operator leaves the 2 s atomic orbital unchanged. Rotation about the C 2 axis leaves the atomic orbital unchanged. Reflections through the sv and sv ’ planes leave the 2 s atomic orbital unchanged. These results correspond to the following row of characters: and this matches those for the symmetry type A 1 in the C 2 v character table. We therefore label the 2 s atomic orbital on the oxygen atom in water as an a 1 orbital.

The oxygen 2 px orbital This matches the row of characters for symmetry type B 1 in the C 2 v character table, and the 2 px orbital therefore possesses b 1 symmetry. The oxygen 2 py orbital This corresponds to symmetry type B 2 in the C 2 v character table, and the 2 py orbital is labelled b 2. The oxygen 2 pz orbital Like the 2 s orbital, the 2 pz orbital there-fore has a 1 symmetry.



BH 3

NH 3


CH 4


Molecular orbital theory: BF 3


5. 7 Molecular orbital theory: learning to use theory objectively p-Bonding in CO 2
![p-Bonding in[NO 3] p-Bonding in[NO 3]](http://slidetodoc.com/presentation_image/4243b49e2514ee52a06d029dffc8f1b0/image-35.jpg)
p-Bonding in[NO 3]

SF 6 The valence orbitals of the S atom in SF 6 can be classified as follows: . the 3 s orbital has a 1 g symmetry; . the 3 px, 3 py and 3 pz orbitals are degenerate and the orbital set has t 1 u symmetry. octahedron.

Separate sets of LGOs can therefore be formed from the F 2 s orbitals and from the F 2 p orbitals. Furthermore, the 2 p orbitals fall into two classes: those that point towards the S atom (radial orbitals, diagram 5. 7) and those that are tangential to the octahedron (diagram 5. 8).


Three-centre two-electron interactions In a 3 c-2 e bonding interaction , two electrons occupy a bonding MO which is delocalized over three atomic centres. Consider [HF 2] The bonding in [HF 2] can be described in terms of the interactions of the H 1 s orbital (sg symmetry) with the LGOs of an F--- F fragment. If we assume a relatively large s p separation for fluorine, then sets of LGOs can be constructed as follows: . LGOs formed by combinations of the F 2 s orbitals; . LGOs formed by combinations of the F 2 pz orbitals; . LGOs formed by combinations of the F 2 px and 2 py orbitals.

Although the H 1 s orbital is of the correct symmetry to interact with either of the F---F sg LGOs, there is a poor energy match between the H 1 s orbital and F---F 2 s 2 s combination.


A more advanced problem: B 2 H 6 The structure of B 2 H 6( D 2 h symmetry) is shown in Figure 5. 31. Features of particular interest are that: . despite having only one valence electron, each bridging H atom is attached to two B atoms; . despite having only three valence electrons, each B atom is attached to four H atoms; . the B H bond distances are not all the same and suggest two types of B H bonding interaction.


Bonding pictures for B 2 H 6 which assume either sp 3 or sp 2 hybridized B centres are frequently adopted, but this approach is not entirely satisfactory.
- Slides: 44