CHAPTER 5 BIOENERGETICS THE FLOW OF ENERGY IN

CHAPTER 5 BIOENERGETICS: THE FLOW OF ENERGY IN THE CELL Every cell has four essential needs: § molecular building blocks, § Information to guide all its activities § chemical catalysts called enzymes, § energy to drive the various reactions and processes

CHAPTER 5 BIOENERGETICS: THE FLOW OF ENERGY IN THE CELL 5. 1 The Importance of Energy 5. 11 Cells need energy to cause six different kinds of Changes 5. 12 Most organisms obtain energy either from sunlight or from organic food molecules 5. 13 Energy flows through the biosphere continuously 5. 14 Flow of energy through the biosphere is accompanied by a flow of matter 5. 2 Bioenergetics 5. 21 To understand energy flow, we need to understand systems, heat, and work 5. 22 The first law of thermodynamics tells us that energy is conserved 5. 23 The second law of thermodynamics tells us that reactions have directionality 5. 24 Entropy and free energy are two alternative means of assessing thermodynamic spontaneity
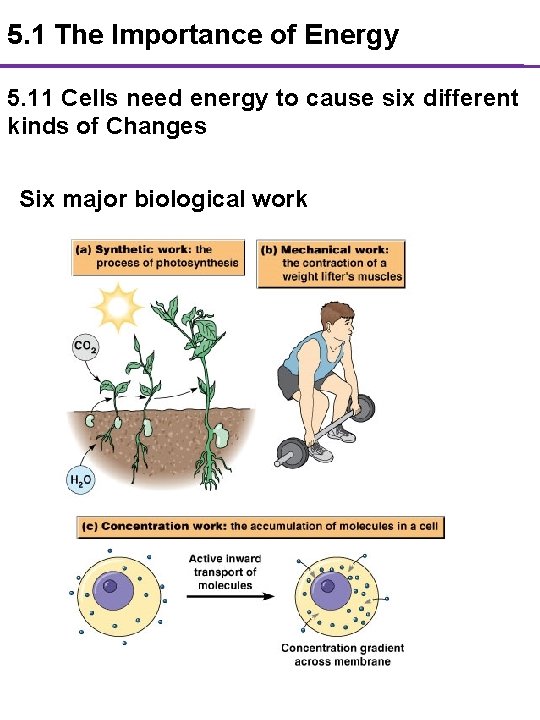
5. 1 The Importance of Energy 5. 11 Cells need energy to cause six different kinds of Changes Six major biological work
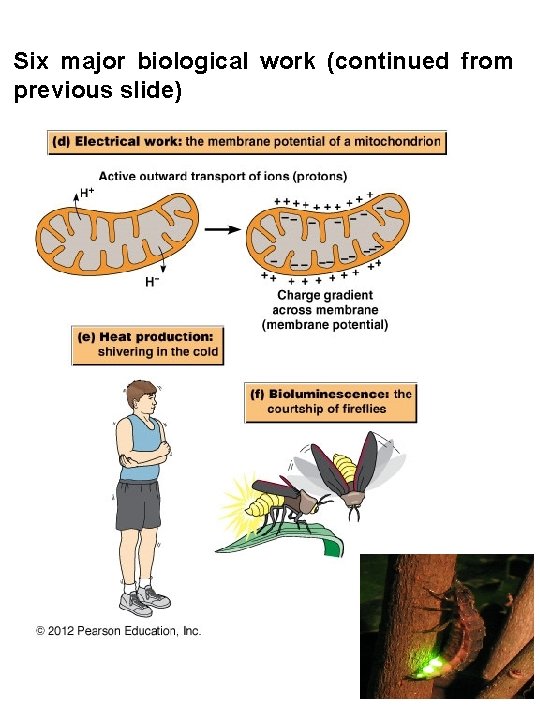
Six major biological work (continued from previous slide)
5. 12 Organisms obtain energy either from sunlight or from organic food molecules Phototrophs (light-feeders): The organisms capable of capturing light energy by means of photosynthesis, then storing the energy in the form of organic molecules like glucose (Plants, algae, and some bacteria). q Photosynthesis: the process in green plants and certain other organisms by which carbohydrates are synthesized from carbon dioxide and water using light as an energy source. Chemotrophs (chemical-feeders): The organism capable of use the energy storing in organic molecules/chemical bond (animals, fungi, and most bacteria). q Fermentation: The chemicals are partially broken down, releasing some of the stored energy (such as glycolysis). q cellular respiration: oxidation-driven movement of electrons with oxygen as a final electron acceptor, during which all the energy stored in chemical bonds is released.

5. 13 Energy flows through the biosphere continuously 5. 14 Flow of energy through the biosphere is accompanied by a flow of matter(matter flow in cycle fashion) Fig. The Flow of Energy Through the Biosphere (1) Phototrophs capture the sun light and use sunlight as energy for the photosynthesis and release oxygen. Phototrophs also obtain nitrogen from soil (from dead animals) and air, convert it into ammonia for synthesis of amino acids, proteins, nucleic acids. .

(2) The chemotrophs take both oxygen and the organic compounds (either from phototrophs or chemotrophs) as indirect energy source. (3) The chemotrophs make carbon dioxide and water that will be used by phototrophs again. Conclusion: §Energy flows unidirectionally from the sun through phototrophs to chemotrophs, whereas matter flows in cycle fashion between phototrophs and chemotrophs. §Some energy is lost as heat during the process using energy to carry out work for both phototrophs and chemotrophs. Thus, total entropy/disorder in the universe is increased.

CHAPTER 5 BIOENERGETICS: THE FLOW OF ENERGY IN THE CELL 5. 1 The Importance of Energy 5. 11 Cells need energy to cause six different kinds of changes 5. 12 Most organisms obtain energy either from sunlight or from organic food molecules 5. 13 Energy flows through the biosphere continuously 5. 14 Flow of energy through the biosphere is accompanied by a flow of matter 5. 2 Bioenergetics 5. 21 To understand energy flow, we need to understand systems, heat, and work 5. 22 The first law of thermodynamics tells us that energy is conserved 5. 23 The second law of thermodynamics tells us that reactions have directionality 5. 24 Entropy and free energy are two alternative means of assessing thermodynamic spontaneity

5. 2 Bioenergetics 5. 21 To understand energy flow, we need to understand systems, heat, and work System: The restricted portion of the universe that one wishes to consider at the moment is called the system: open system and closed system (All living organisms are open system, since they exchange energy freely with their surroundings). Fig. Open and closed systems Surroundings: The rest of the universe that is not occupied by the system is called the surroundings.

Heat and work: The two ways for the exchange of energy between a system and its surroundings. Heat: is the energy transfer from one place to another as a result of a temperature difference between the two places. Work: is the energy used to drive any process other than heat flow. Calorie: is a unit of energy, which is the amount of energy required to warm 1 gram water for increase of 1 degree (centigrade) at a pressure of 1 atmosphere. 1 cal = 4. 184 Joule (J) , or 1 J = 0. 239 cal.

5. 22 The first law of thermodynamics tells us that energy is conserved
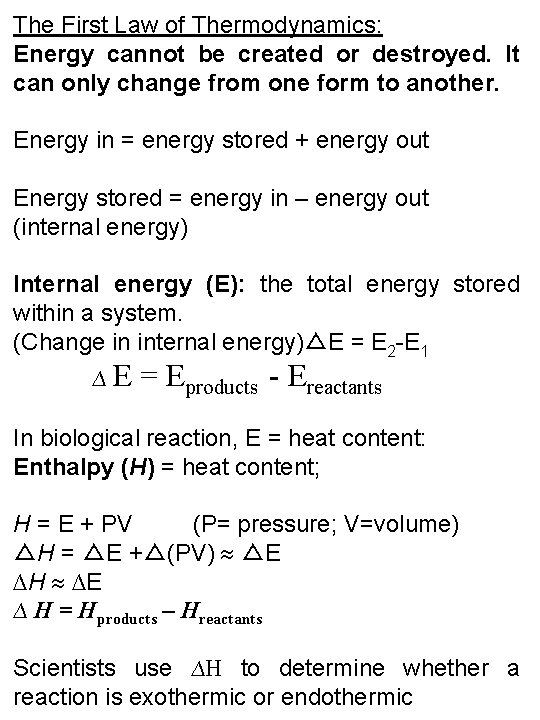
The First Law of Thermodynamics: Energy cannot be created or destroyed. It can only change from one form to another. Energy in = energy stored + energy out Energy stored = energy in – energy out (internal energy) Internal energy (E): the total energy stored within a system. (Change in internal energy)△E = E 2 -E 1 ∆E = Eproducts - Ereactants In biological reaction, E = heat content: Enthalpy (H) = heat content; H = E + PV (P= pressure; V=volume) △H = △E +△(PV) △E ∆H ∆E ∆ H = Hproducts – Hreactants Scientists use ∆H to determine whether a reaction is exothermic or endothermic
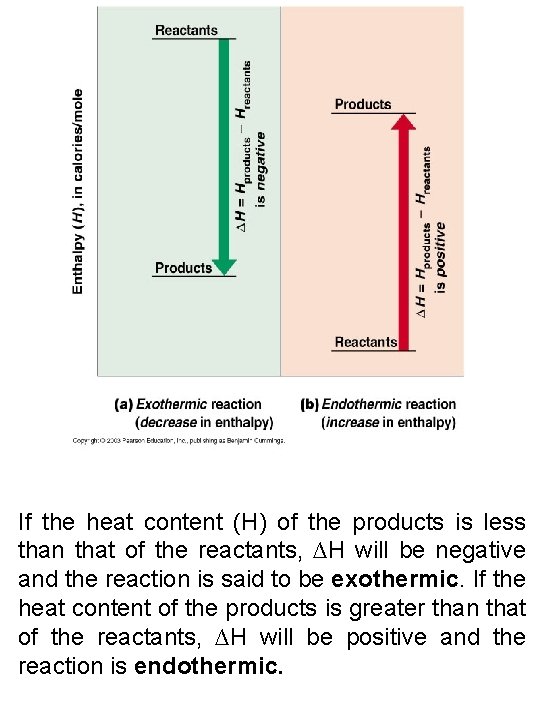
If the heat content (H) of the products is less than that of the reactants, ∆H will be negative and the reaction is said to be exothermic. If the heat content of the products is greater than that of the reactants, ∆H will be positive and the reaction is endothermic.

5. 23 The Second Law of Themodynamics tells US that Reactions Have Directionality Thermodynamically spontaneous reactions: Energy flows from the sun through phototrophs to chemotrophs (can the energy flow back to the sun spontaneously? ) Some energy in any process or reaction in a system will be lost as heat, such as during cooking (can the lost heat go back to the reaction complex spontaneously? ). Put a drops of dye in water, it will diffuse in water (can the diffused dye come back spontaneously to a drops of dye? ) A room will become more disorganized as time elapses (can the disorganized room will go back to the organized room spontaneously? ) As a result, disorder in the universe is continuously increased.

before summer after Summer before summer Could your room be like this if nobody clean your room during the summer? Figure (supple). Entropy is in action
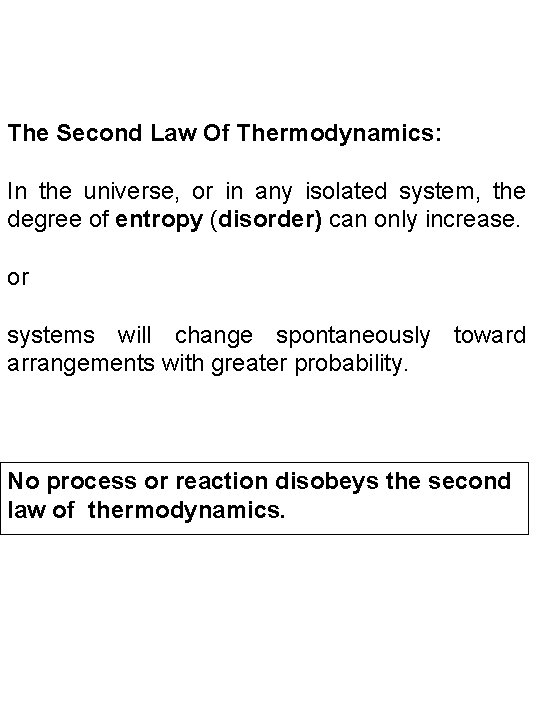
The Second Law Of Thermodynamics: In the universe, or in any isolated system, the degree of entropy (disorder) can only increase. or systems will change spontaneously toward arrangements with greater probability. No process or reaction disobeys the second law of thermodynamics.
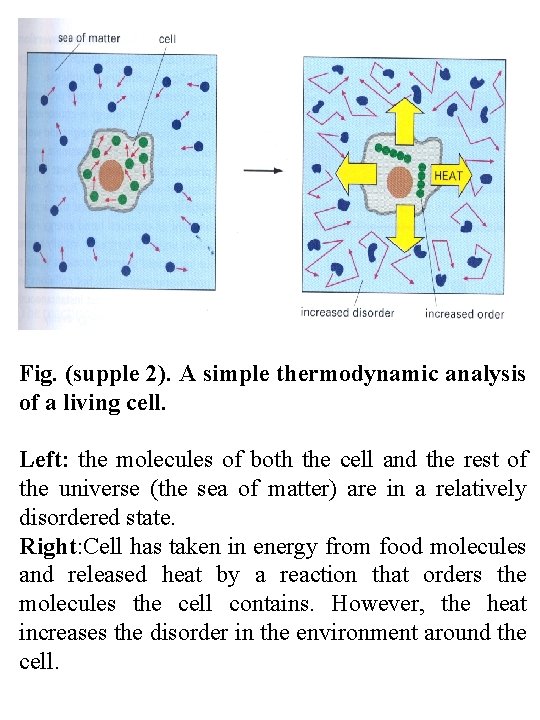
Fig. (supple 2). A simple thermodynamic analysis of a living cell. Left: the molecules of both the cell and the rest of the universe (the sea of matter) are in a relatively disordered state. Right: Cell has taken in energy from food molecules and released heat by a reaction that orders the molecules the cell contains. However, the heat increases the disorder in the environment around the cell.

5. 24 Entropy and free energy are two alternative means of assessing thermodynamic spontaneity Entropy (S) : The unit or quantity that we use to measure the disorder. For any system, the change in entropy, ∆S, represents a change in the degree of randomness or disorder of the components of the system. The greater the disorder, the greater the entropy. All processes or reactions that occur spontaneously result in an increase in the total entropy of the universe (∆Suniverse is always positive). Free Energy (G) The amount of energy actually available to break and subsequently form other chemical bond, or in a more general sense, free energy is defined as the energy available to do work in any system (G is equal to H minus S) H = G + S, H = G + TS, G = H-TS; ∆G = ∆H - T∆S. △G = Gproducts- Greactants
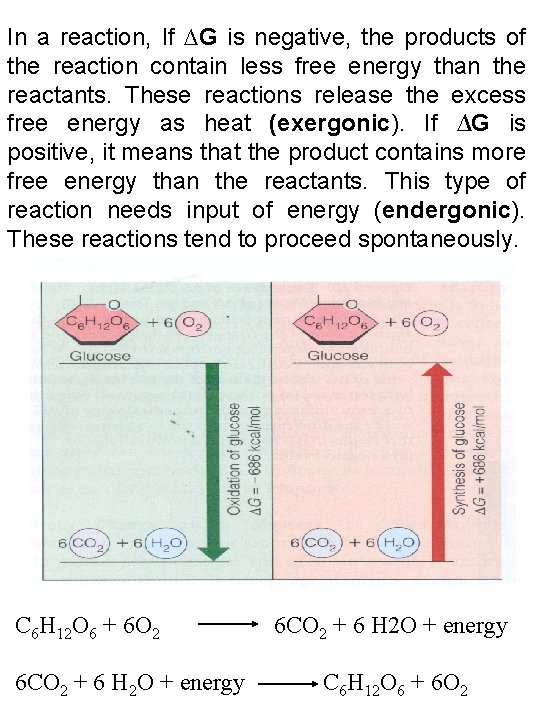
In a reaction, If ∆G is negative, the products of the reaction contain less free energy than the reactants. These reactions release the excess free energy as heat (exergonic). If ∆G is positive, it means that the product contains more free energy than the reactants. This type of reaction needs input of energy (endergonic). These reactions tend to proceed spontaneously. C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O + energy C 6 H 12 O 6 + 6 O 2

∆G’ is ∆G in the standard condition For comparison and convenience in reporting, scientists set standard condition for measuring ∆G (∆G’): For systems consisting of dilute aqueous solutions, the standard condition: 25 o. C 1 atmosphere all products and reactants present in their most stable forms at a standard concentration of 1 mol/L

Summary (Bioenergetics): (1) What are systems, heat, and work (2) The first law of thermodynamics: energy cannot be created or destroyed. It can only be changed from one form to another. The total energy stored within a system is called internal energy (E). Siminar to E, enthalpy or heat content (H) has been used in biological reactions to determine whether a reaction is exothermic or endothermic For a chemical reaction: H = Hproducts-Hreactants If H is negative, it means that the reaction is exothermic. (3) The Second law of thermodynamics: In the universe, or in any isolated system, the degree of disorder can only increase. Entropy (S): For a closed thermodynamic system, a quantitative measure of the amount of thermal energy represents a change in the degree of disorder of the components of the system. Free energy (G) is the energy actually to do work: Free Energy (G): G = H-T S If G is negative, the reactions or processes are exergonic; processes are endergonic. `

Key Concepts What is energy unit? What is internal energy, enthalpy, entropy, free energy? What are thermodynamic first law and second law? Can you give several examples? What do exothermic, endothermic, exergonic, and endergonic mean? Can you give several examples? What are energy conservative and spontaneous reactions? Do they mean the same thing?
- Slides: 22