Chapter 5 Atomic Structure The Periodic Table College
Chapter 5 Atomic Structure & The Periodic Table College Prep Chemistry Mrs. Lips WOC The Atom Ancient Ideas

The Atom: From Philosophical Idea to Scientific Theory o Democritus (Greek philospher; 450 BCE) n First suggested the idea that atoms existed but had no experimental support o Atoms: the smallest particle of an element that retains the properties of that element. n (Greek: atomos = indivisible)

John Dalton (1766 -1844) n English school teacher n Performed experiments and came up with 5 hypotheses to explain his results (Dalton’s Atomic Theory)
Dalton’s Atomic Theory o 1. All elements are composed of tiny indivisible particles called atoms. o 2. Atoms of the same element are identical. The atoms of one element are different from those of another.
Dalton’s Atomic Theory (cont’d) o 3. Atoms of different elements can combine with one another in simple whole number ratios. n H 2 O C 12 H 22 O 11 NOT H 2. 5 O¾ o 4. Chemical reactions occur when atoms are separated, joined or rearranged. Atoms are not changed into atoms of another!

Dalton’s Atomic Theory o 5. Atoms can not be subdivided. n Today, we know they can be divided into subatomic particles
Dalton’s atom o A simple sphere with no internal structure o The way compounds were held together was poorly understood
How small is an atom? o Average atom size: n Diameter = 1 x 10 -8 cm n Mass = 1 x 10 – 23 g o 100, 000 copper atoms in a row would = 1 cm in length! Atomic Size

Changes to Dalton’s Atomic Theory o Most of Dalton’s Atomic Theory is accepted o One major revision includes the idea that atoms have smaller parts… o o There are 3 parts to an atom…. 1. electrons 2. protons 3. neutrons

The Electron (e-) o Discovered by J. J. Thomson (1897) n “Cathode Ray” n Negatively charged subatomic particles called electrons responsible for the ray were attracted to the positive end of a magnet n Plum Pudding Model
The Electron (e-) continued o Robert Milikan (1916) n Determined the charge and mass of an electron o 1 unit of negative charge o mass about 1800 times smaller than the mass of a hydrogen atom
2. The Proton (p+) o Discovered by Goldstein (1886) n Positively charged subatomic particles called protons n 1, 840 times heavier than an electron
3. The Neutron (no) o discovered by James Chadwick (1932) n Subatomic particles with no charge called neutrons n Mass is nearly the same as a proton
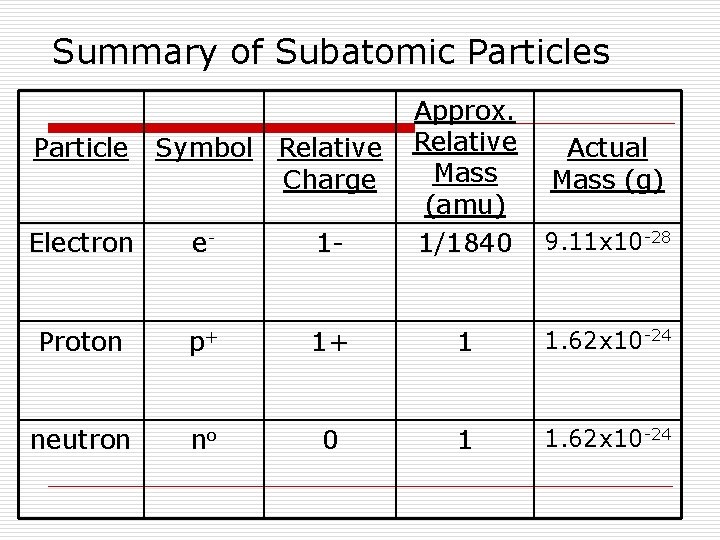
Summary of Subatomic Particles Electron e- 1 - Approx. Relative Mass (amu) 1/1840 Proton p+ 1+ 1 1. 62 x 10 -24 neutron no 0 1 1. 62 x 10 -24 Particle Symbol Relative Charge Actual Mass (g) 9. 11 x 10 -28
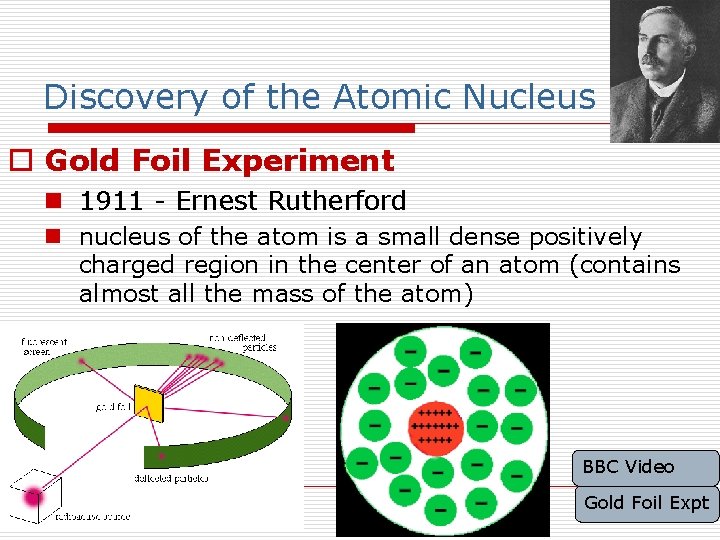
Discovery of the Atomic Nucleus o Gold Foil Experiment n 1911 - Ernest Rutherford n nucleus of the atom is a small dense positively charged region in the center of an atom (contains almost all the mass of the atom) BBC Video Gold Foil Expt
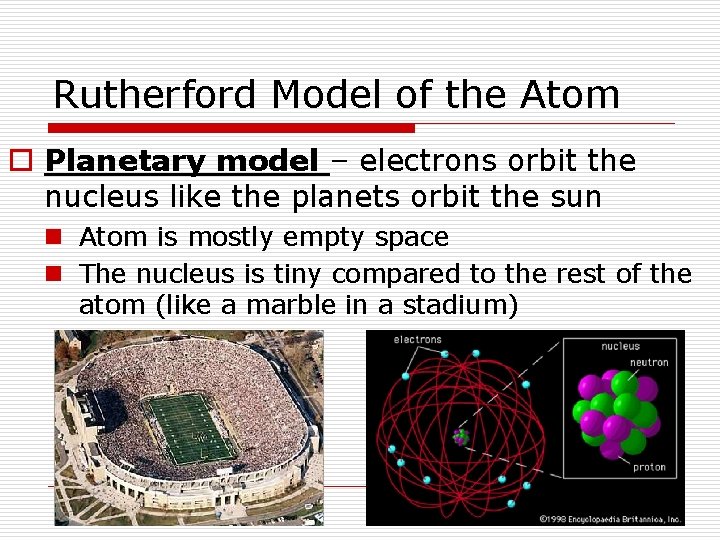
Rutherford Model of the Atom o Planetary model – electrons orbit the nucleus like the planets orbit the sun n Atom is mostly empty space n The nucleus is tiny compared to the rest of the atom (like a marble in a stadium)
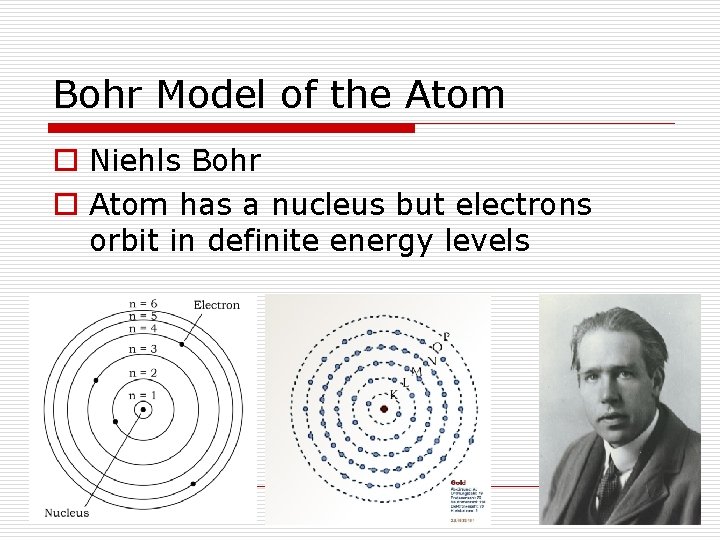
Bohr Model of the Atom o Niehls Bohr o Atom has a nucleus but electrons orbit in definite energy levels
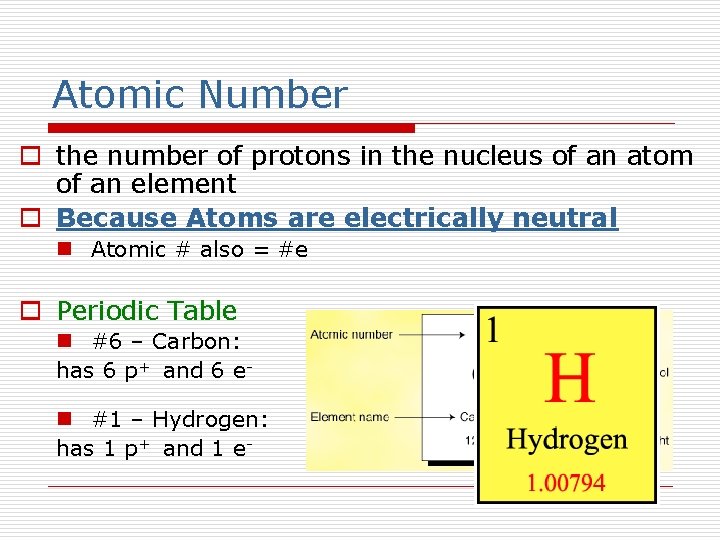
Atomic Number o the number of protons in the nucleus of an atom of an element o Because Atoms are electrically neutral n Atomic # also = #e o Periodic Table n #6 – Carbon: has 6 p+ and 6 en #1 – Hydrogen: has 1 p+ and 1 e-
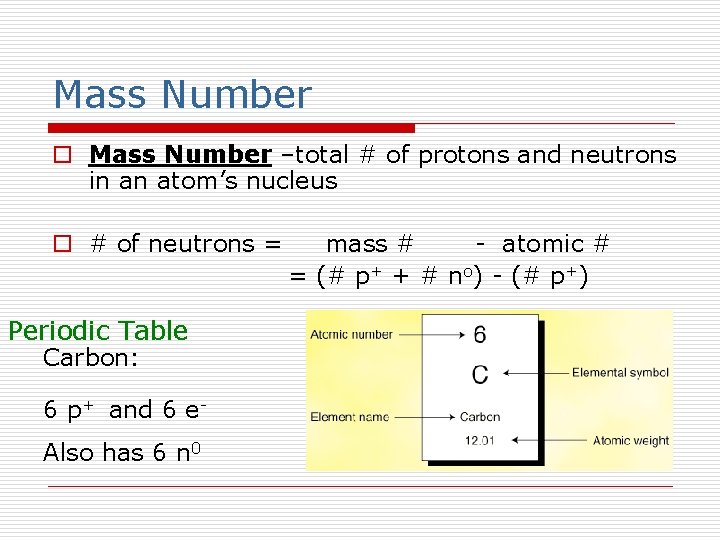
Mass Number o Mass Number –total # of protons and neutrons in an atom’s nucleus o # of neutrons = Periodic Table Carbon: 6 p+ and 6 e. Also has 6 n 0 mass # - atomic # = (# p+ + # no) - (# p+)
Determining # of particles o Atomic # = number of protons o Number of protons = number of electrons (in a neutral atom) o Mass number = # of protons + # of neutrons
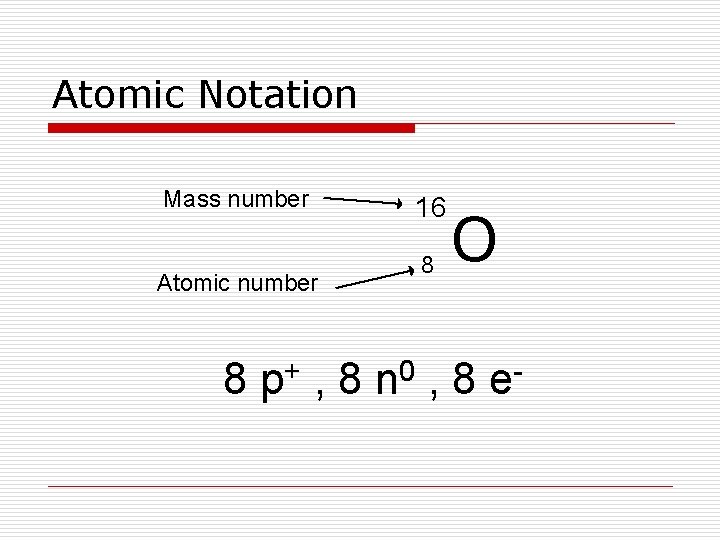
Atomic Notation Mass number Atomic number 16 8 O 8 p+ , 8 n 0 , 8 e -

Practice… Element: _______ Atomic #: _______ Mass #: ________ Protons: ________ Neutrons: _______ Electrons: _______ More Practice
Isotopes o Definition – atoms that have the same number of protons but different numbers of neutrons o Identified by mass number o Atomic # (# of protons) stays the same o Ex) Carbon – has 3 isotopes n 1) Carbon – 12 n 2) Carbon – 13 n 3) Carbon – 14
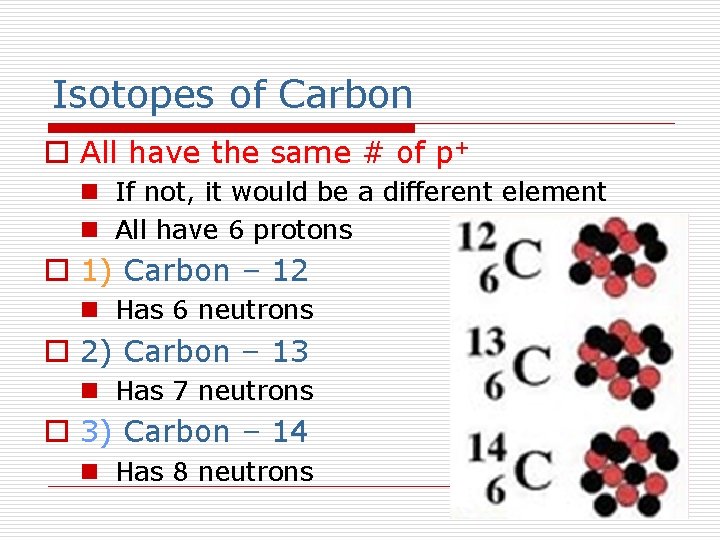
Isotopes of Carbon o All have the same # of p+ n If not, it would be a different element n All have 6 protons o 1) Carbon – 12 n Has 6 neutrons o 2) Carbon – 13 n Has 7 neutrons o 3) Carbon – 14 n Has 8 neutrons
Atomic Mass o Definition = weighted average mass of the atoms in a naturally occurring sample of the element n Units = amu o Equal to 1/12 the mass of a carbon-12 atom n This is the mass that is on the periodic table n Based on the mass and abundance of each isotope
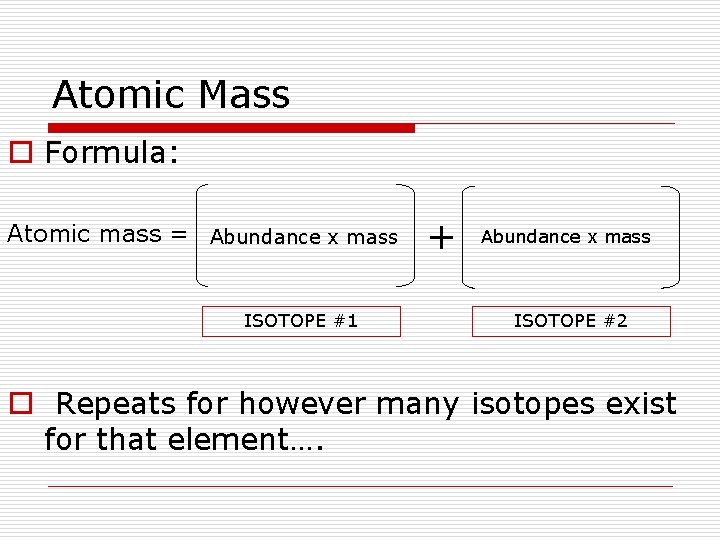
Atomic Mass o Formula: Atomic mass = Abundance x mass ISOTOPE #1 + Abundance x mass ISOTOPE #2 o Repeats for however many isotopes exist for that element….
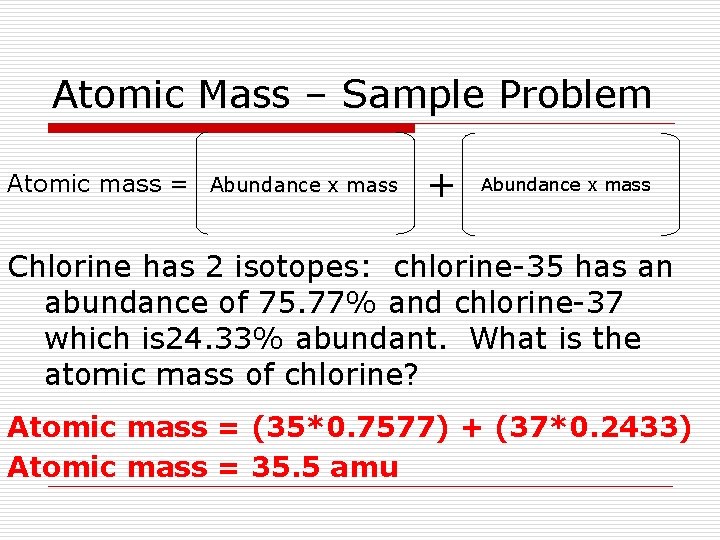
Atomic Mass – Sample Problem Atomic mass = Abundance x mass + Abundance x mass Chlorine has 2 isotopes: chlorine-35 has an abundance of 75. 77% and chlorine-37 which is 24. 33% abundant. What is the atomic mass of chlorine? Atomic mass = (35*0. 7577) + (37*0. 2433) Atomic mass = 35. 5 amu
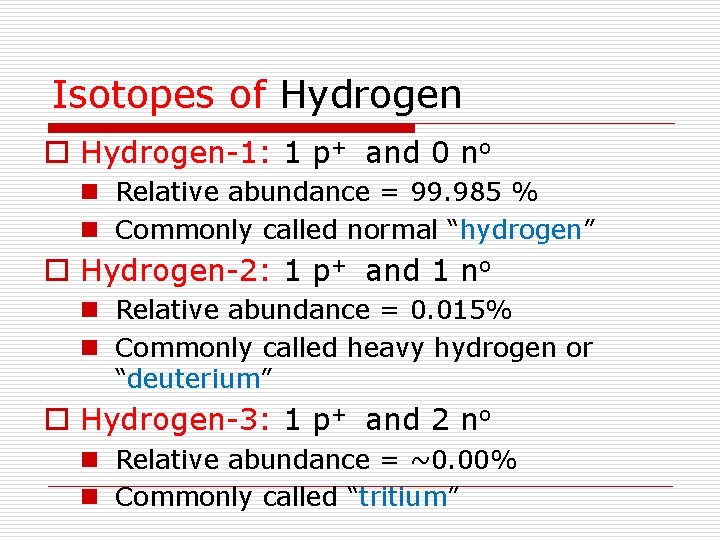
Isotopes of Hydrogen o Hydrogen-1: 1 p+ and 0 no n Relative abundance = 99. 985 % n Commonly called normal “hydrogen” o Hydrogen-2: 1 p+ and 1 no n Relative abundance = 0. 015% n Commonly called heavy hydrogen or “deuterium” o Hydrogen-3: 1 p+ and 2 no n Relative abundance = ~0. 00% n Commonly called “tritium”
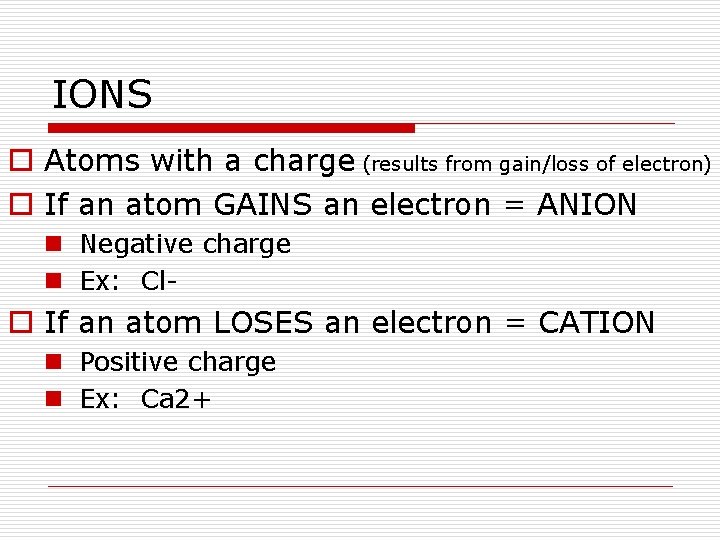
IONS o Atoms with a charge (results from gain/loss of electron) o If an atom GAINS an electron = ANION n Negative charge n Ex: Cl- o If an atom LOSES an electron = CATION n Positive charge n Ex: Ca 2+
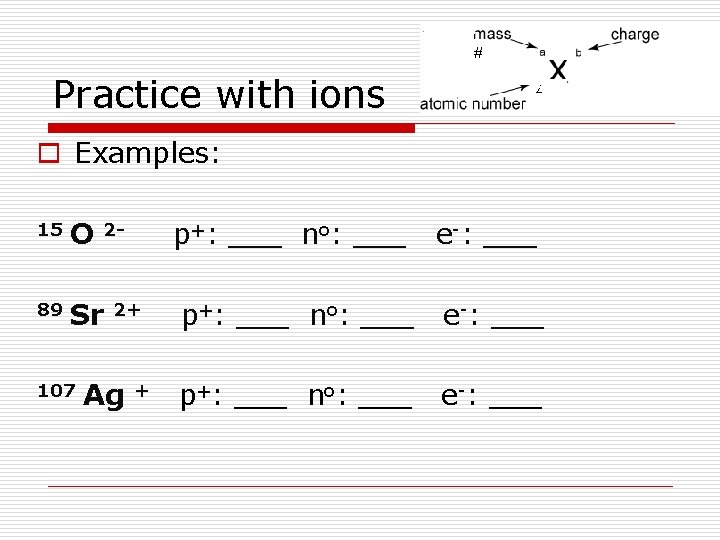
Practice with ions o Examples: 15 O 89 Sr 107 2 - 2+ Ag + p+: ___ no: ___ e-: ___
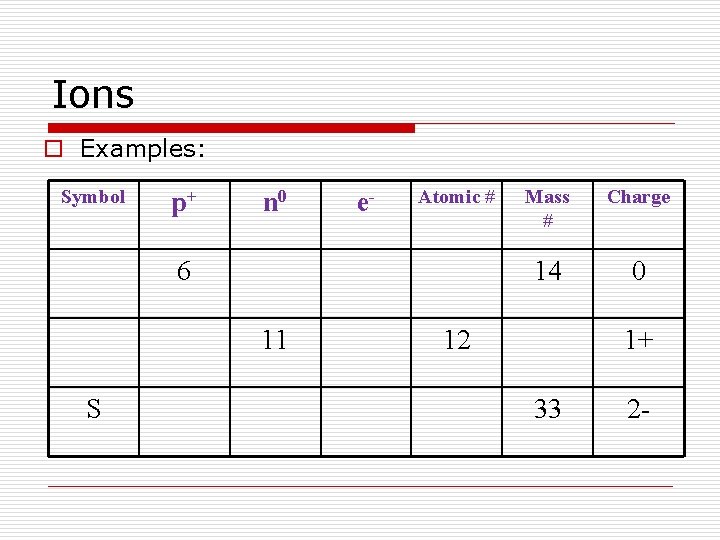
Ions o Examples: Symbol p+ n 0 e- Atomic # 6 11 S Mass # Charge 14 0 12 1+ 33 2 -

CH 5 EOC Questions o Answer the following in your composition book o Questions found on page 129 o #33, 36, 38 -43, 45 -46, 48 -49, 53, 57
- Slides: 33