Chapter 5 Atomic Structure and the Periodic Table

Chapter 5 Atomic Structure and the Periodic Table

Just How Small is an Atom? You don’t need to write. A penny contains about 2. 4 × 1022 atoms. n A speck 0. 1 mm in diameter (about half the size of a period at the end of the sentence) requires one million atoms. n It would require a million atoms, edge to edge, to match the thickness of a page of paper. n

History of the Development of Atomic Models A. Democritus (400 B. C. ) Ø 1 st suggested matter is made of atoms Ø atom- means “indivisible”

John Dalton You don’t need to write. n John Dalton (1766 -1844), was an English schoolteacher. n Performed experiments to test his atomic theory. n Formulated hypothesis and theories to explain his observations.

History of the Development of Atomic Models B. Dalton (1766 -1844) Ø Dalton’s Atomic Theory 1. All elements are composed of tiny indivisible particles called atoms Is this still true? YES

Dalton’s Atomic Theory 2. Atoms of same element are identical. The atoms of any one element are different from those of any other element. Is this still true? v atoms of the same element can have different masses (isotopes)

Dalton’s Atomic Theory 3. Atoms form compounds by combining in whole number ratios Is this still true? v YES: Law of Definite Proportions

Dalton’s Atomic Theory 4. Chemical reactions occur when atoms are separated, joined or rearranged. Atoms of one element can never change into another element. Is this still true? No, These changes CAN occur in nuclear reactions!

Structure of the Atom § Nucleus: § Electron Cloud: – contains protons and neutrons -contains electrons -takes up most of space – takes up very little space

Discovery of Nucleus C. Rutherford (1871 -1937) discovered the nucleus by shooting alpha particles (have positive charge) at a very thin piece of gold foil – he predicted that the particles would go right through the foil at some small angle

Discovery of Nucleus

Discovery of Nucleus n some particles (1/8000) bounced back from the foil n this meant there must be a “powerful force” in the foil to hit particle back Predicted Results Actual Results

Subatomic Particles D. J. J. Thomson (1856 -1904) – discovered electrons in atoms; his model was of a positive sphere with e- embedded in it. E. Milliken (1868 -1953) found the mass of the electron F. Goldstein found protons in 1886 G. Chadwick (1891 -1974) found the neutron

Discovery of Electron You don’t need to write. n n n resulted from scientists passing electric current through gases to test conductivity used cathode-ray tubes noticed that when current was passed through a glow (or “ray”) was produced

Discovery of Electron This led scientists to believe there were negatively charged particles inside the cathode ray

Properties of Subatomic Particles Relative Particle Symbol electrical charge Relative Actual mass (g) Electron e- 1 - 1/1840 9. 11 × 10 -28 Proton p+ 1+ 1 1. 67 × 10 -24 Neutron n 0 0 1 1. 67 × 10 -24
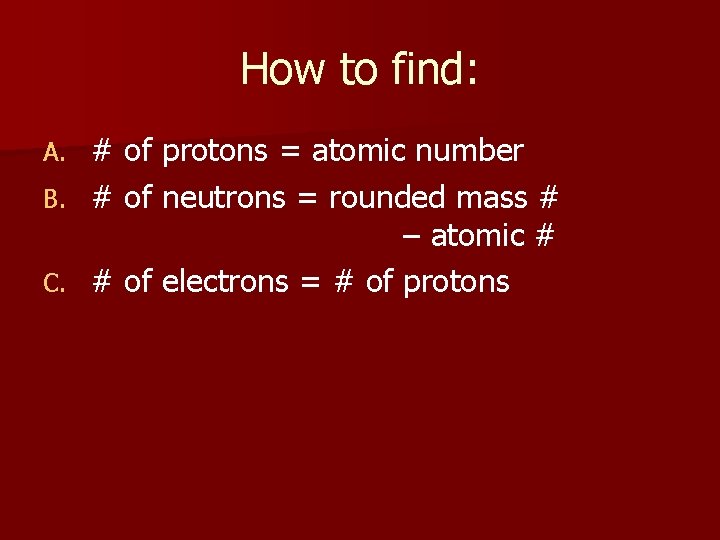
How to find: # of protons = atomic number B. # of neutrons = rounded mass # – atomic # C. # of electrons = # of protons A.

1. Chemical Symbols a) Printed: 1 st letter capital, 2 nd letter lower case b) Represents one atom of an element Fe Cl B

2. Important principles about the atom a) b) c) d) All atoms are electrically neutral (p+ = e-) Nearly all mass is in the nucleus Lots of space between nucleus and e. Every atom of the same element has the same # of p+

3. Atomic Number is the # of p+ Element Atomic # # of Protons Carbon 6 6 Phosphorus 15 15 Gold 79 79

4. Mass Number is the # of p+ + n 0 Mass Number Atomic Number 12 6 C Element Symbol

5. Isotope of an element- same # p+, different # of n 0 (Same atomic #, different mass #) 6. Atomic mass unit (amu)- defined as 1/12 of the mass of a Carbon-12 atom

7. Atomic Mass- mass averaged of all the isotope of an element.

Mass Number 8. Isotopic Name: Carbon-12 or Carbon-14 a. 10 Ne b. Hydrogen-3 21 p+ 10 e- 10 n 0 11 p+ 1 e- 1 n 0 2 c. Magnesium-27 27 12 Mg

Isotope Lab Avg. mass = Mass (of that type of veggie) # of pieces of that type of veggie % abundance = # of pieces of that veggie × 100 Total # of pieces of all veggies

Bell Work 9/21 The nucleus consist of ______ and _______. The ______ number of an atom gives the number of protons. The _____ number gives you how many protons and neutrons are in the nucleus. _________ 23 _____ 11 Na Copper-63 Atoms that have the same _______ but different numbers of neutrons are ____ of the same element. Since isotopes have different numbers of neutrons, they have a different _______ ______.

C. Nuclear Symbols n Chlorine-37 – atomic #: 17 – mass #: 37 – # of protons: 17 – # of electrons: 17 – # of neutrons: 20

Nuclear Symbol Examples 35 17 Cl Atomic Number 17 Mass Number 35 Mg Number of Protons 17 Number of Electrons 17 Number of Neutrons 18 27 12 Atomic Number 12 Mass Number 27 Number of Protons 12 Number of Electrons 12 Number of Neutrons 15

Average Atomic Mass n weighted average of all naturally occuring isotopes n on the Periodic Table n round to 2 decimal places Avg. Atomic Mass

Average Atomic Mass n EX: Calculate the avg. atomic mass of oxygen if its abundance in nature is 99. 76% 16 O, 0. 04% 17 O, and 0. 20% 18 O. Avg. Atomic Mass 16. 00 amu

Average Atomic Mass n EX: Calculate the avg. atomic mass of oxygen if its abundance in nature is 99. 76% 16 O, 0. 04% 17 O, and 0. 20% 18 O. Avg. Atomic Mass 16. 00 amu

E. Average Atomic Mass n EX: Find chlorine’s average atomic mass if approximately 8 of every 10 atoms are chlorine 35 and 2 are chlorine-37. Avg. Atomic Mass 35. 40 amu
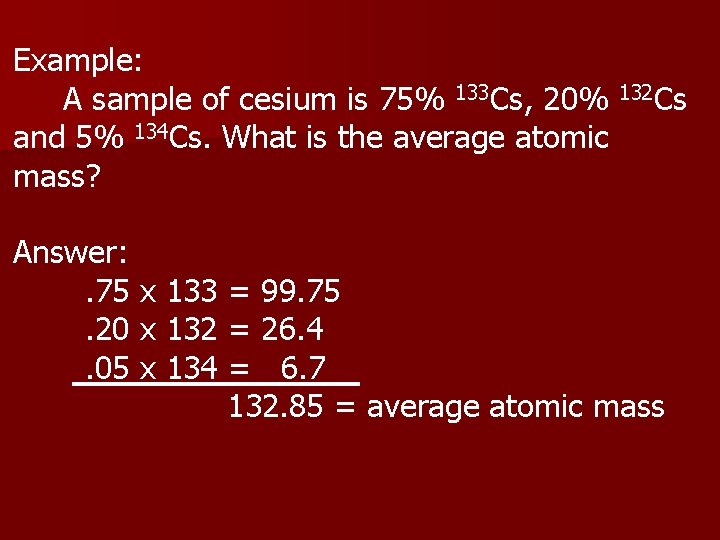
Example: A sample of cesium is 75% 133 Cs, 20% 132 Cs and 5% 134 Cs. What is the average atomic mass? Answer: . 75 x 133 = 99. 75. 20 x 132 = 26. 4. 05 x 134 = 6. 7 132. 85 = average atomic mass
- Slides: 33