CHAPTER 5 3 THE PERIODIC TABLE Integrated Science
CHAPTER 5. 3 THE PERIODIC TABLE Integrated Science

SECTION OBJECTIVES SECTION 5. 3 - REPRESENTATIVE GROUPS After this presentation you will be able to: 1. Relate the number of valence electrons to groups in the periodic table and to elements in those groups 2. Predict the reactivity of some elements based on their locations within a group 3. Explain trends in properties across and throughout the periodic table 4. Identify some properties of common A group elements
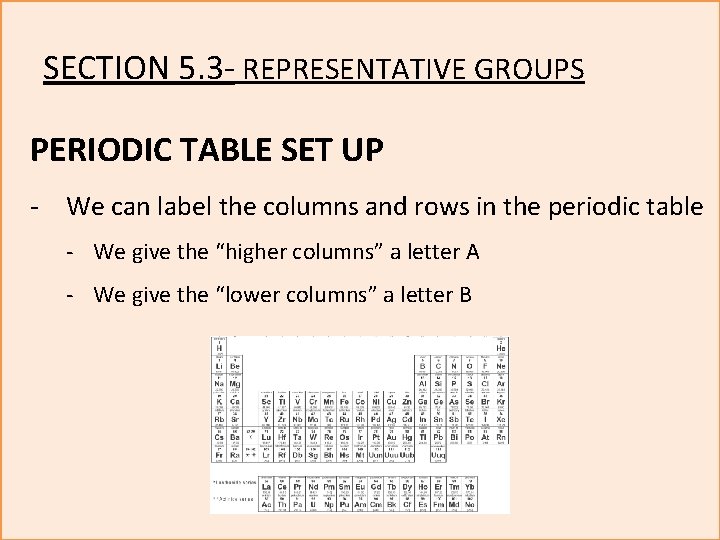
SECTION 5. 3 - REPRESENTATIVE GROUPS PERIODIC TABLE SET UP - We can label the columns and rows in the periodic table - We give the “higher columns” a letter A - We give the “lower columns” a letter B

SECTION 5. 3 - REPRESENTATIVE GROUPS VALENCE ELECTRONS - We mentioned that the rows are cut off at certain places because of their electron configuration - Alike elements then line up in columns - Same columns = same properties

SECTION 5. 3 - REPRESENTATIVE GROUPS VALENCE ELECTRONS - Valence Electrons are electrons that occupy the highest energy level of an atom • These outer electrons are the ones that partake in chemical reactions - Elements in a group have similar properties because they have the same number of valence electrons
ELECTRONS IN ENERGY LEVELS • Remember we can fit 2 electrons on each ring
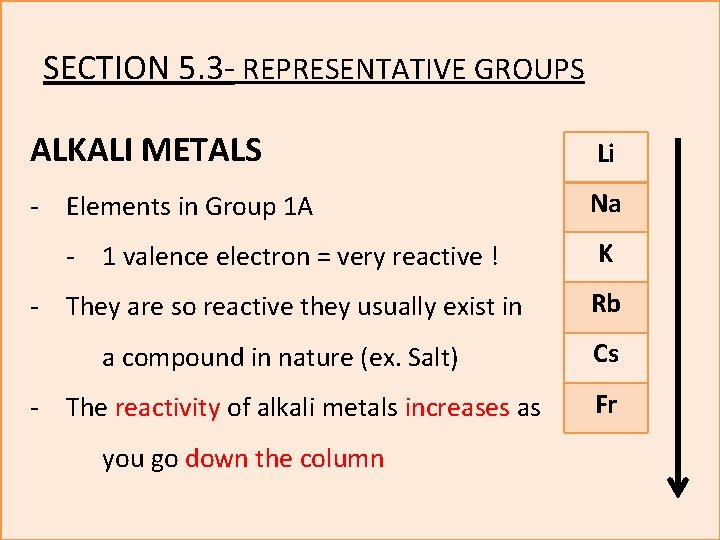
SECTION 5. 3 - REPRESENTATIVE GROUPS ALKALI METALS Li - Elements in Group 1 A Na - 1 valence electron = very reactive ! K - They are so reactive they usually exist in Rb a compound in nature (ex. Salt) Cs - The reactivity of alkali metals increases as Fr you go down the column
SECTION 5. 3 - REPRESENTATIVE GROUPS ALKALINE EARTH METALS Be - Elements in Group 2 A Mg - 2 valence electron = somewhat reactive - Reactivity differences can be seen with how the metals react with water Ca Sr Ba Ra
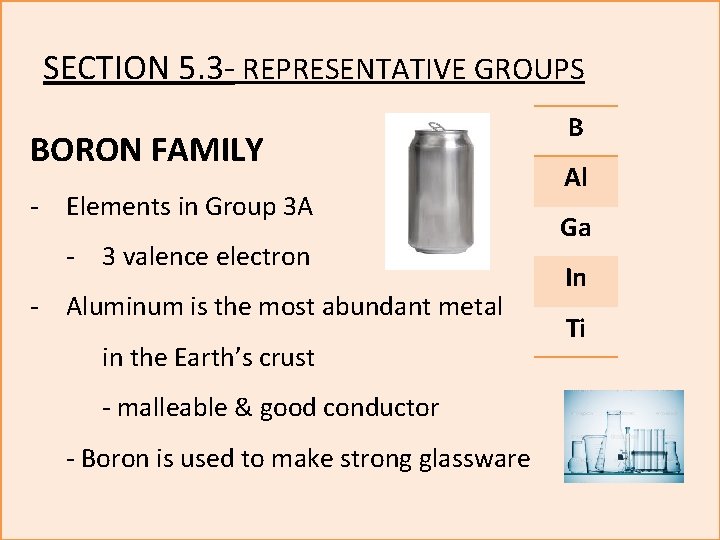
SECTION 5. 3 - REPRESENTATIVE GROUPS BORON FAMILY - Elements in Group 3 A - 3 valence electron - Aluminum is the most abundant metal in the Earth’s crust - malleable & good conductor - Boron is used to make strong glassware B Al Ga In Ti
SECTION 5. 3 - REPRESENTATIVE GROUPS CARBON FAMILY C - Elements in Group 4 A - 4 valence electron Si - Elements take on more metallic properties as you down the group - Life on Earth would not exist without Carbon Ge Sn Pb - Silicon is the second most abundant element in the Earth’s crust

SECTION 5. 3 - REPRESENTATIVE GROUPS NITROGEN FAMILY - Elements in Group 5 A - 5 valence electron N P As - Elements have a wide range of physical properties Sb - Nitrogen is used in fertilizer and is Bi the most abundant gas in the air - Fertilizers also contain phosphorous

SECTION 5. 3 - REPRESENTATIVE GROUPS OXYGEN FAMILY - Elements in Group 6 A - 6 valence electron - Oxygen is necessary for life because it helps release the energy stored in food - Sulfur is found in volcanoes & geysers O S Se Te Po

SECTION 5. 3 - REPRESENTATIVE GROUPS HALOGENS - Elements in Group 7 A - 7 valence electron - Despite their physical differences, halogens have similar chemical properties - they are very reactive ! F Cl Br I At - Chlorine kills bacteria/in bleach - Iodine is needed for the thyroid gland to function

SECTION 5. 3 - REPRESENTATIVE GROUPS NOBLE GASES - Elements in Group 8 A - 8 valence electron * Except helium which has 2 He Ne Ar Kr - Colorless & odorless Xe - Extremely unreative - Release different colors when electric current passes through them
- Slides: 14