Chapter 5 1 History of the Periodic Table

Chapter 5 -1 History of the Periodic Table

1860 �There was no method for accurately determining an element’s atomic mass or the number of atoms of an element in a particular compound. �Stanislao Cannizzaro presented a method to measure the relative masses of atoms. Also initiated the development of the periodic table.
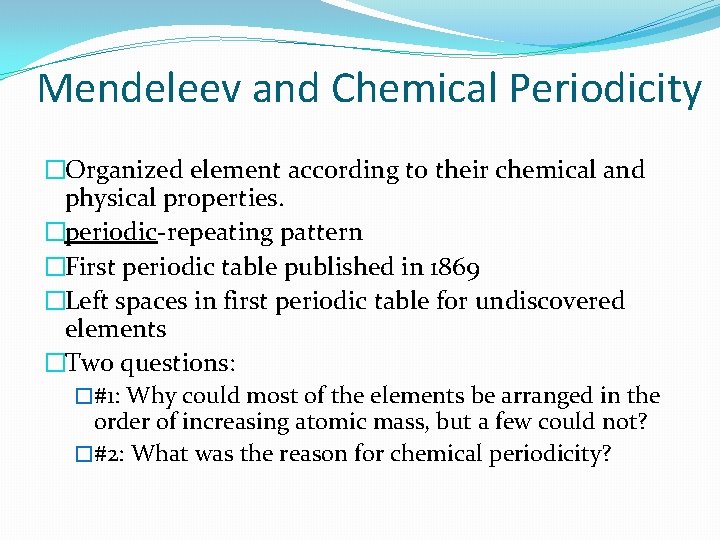
Mendeleev and Chemical Periodicity �Organized element according to their chemical and physical properties. �periodic-repeating pattern �First periodic table published in 1869 �Left spaces in first periodic table for undiscovered elements �Two questions: �#1: Why could most of the elements be arranged in the order of increasing atomic mass, but a few could not? �#2: What was the reason for chemical periodicity?
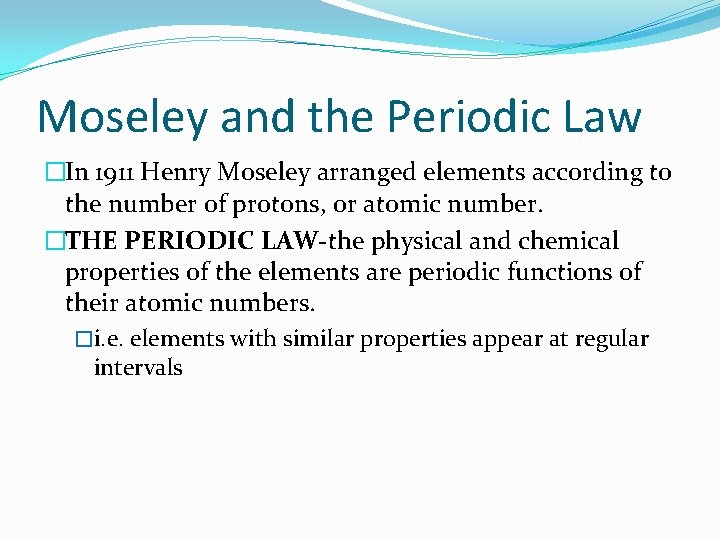
Moseley and the Periodic Law �In 1911 Henry Moseley arranged elements according to the number of protons, or atomic number. �THE PERIODIC LAW-the physical and chemical properties of the elements are periodic functions of their atomic numbers. �i. e. elements with similar properties appear at regular intervals

The Modern Periodic Table �The periodic table is an arrangement of the elements in order of their atomic numbers so that elements with similar properties fall in the same column or group.

Noble Gases �In 1775 the English scientist Henry Cavendish analyzed a sample of air. He found that after all the substances then known to be in air were accounted for, a tiny part of the original sample remained. �Using two different methods to remove all known gases from air, Sir William Ramsay and Lord Rayleigh were able to announce in 1894 that they had found a monatomic, chemically inert gaseous element that constituted nearly 1 percent of the atmosphere; they named it argon. � The following year, Ramsay liberated another inert gas which proved to be helium. (Helium had previously been discovered and was known to be a component of the sun but this was the first time it was shown to exist on Earth. )

The Lanthanides �Early 1900’s the lanthanides are placed on the periodic table. �Atomic numbers 58 (cerium)-71 (lutetium). �New name –lanthanoids because the suffix "-ide" generally indicates negative ions.

Home fun �Pg 127 q 1 -4

s block - Group 1 - Alkali Metals �Main group elements �Silvery, soft and highly reactive. �Not found in nature as free elements but found in compounds instead. �React strongly with water to produce hydrogen gas (H 2) and aqueous solutions of alkalis. �Low melting point. Melting point lowers as we move down the group.

s-block group 2 Alkaline-earth metals �Harder, denser and stronger than alkali metals �Higher melting points than alkali metals �Still highly reactive, not found in nature…but less reactive than group 1 metals

Hydrogen and Helium �Placed above the Group 1 elements in the periodic table �Hydrogen’s properties do not resemble those of the other group 1 elements �Helium is placed in group 18 because with 2 electrons, its highest energy level is full �It is a noble gas…unreactive, inert.

d block: groups 3 -12 �Transition metals �Good conductors of electricity and have a high luster �Less reactive than groups 1 and 2 �Some do not easily form compounds �Elements in these groups do NOT necessarily have the same outer electron configurations.

p-block Groups 13 -18 �Main group elements �Noble gases: group 18, nonreactive gases �Halogens: group 17, highly reactive gases �Metalloids: semi-conductors, brittle solids with properties of metals and nonmetals �Metals: harder/denser than s-block metals, but softer than d-block metals, sufficiently reactive
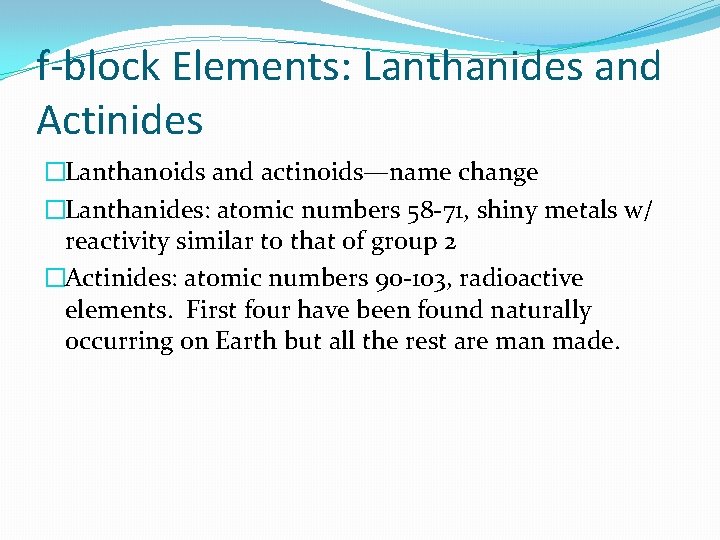
f-block Elements: Lanthanides and Actinides �Lanthanoids and actinoids—name change �Lanthanides: atomic numbers 58 -71, shiny metals w/ reactivity similar to that of group 2 �Actinides: atomic numbers 90 -103, radioactive elements. First four have been found naturally occurring on Earth but all the rest are man made.

- Slides: 15