Chapter 49 Antineoplastic Drugs Department of pharmacology Liu





































- Slides: 47

Chapter 49 Antineoplastic Drugs Department of pharmacology Liu xiaokang(刘小康) 2010, 3

Categories: • 1) Antimetabolites: a) Folic Acid Analogs (Methotrexate, MTX). b) Pyrimidine analogs (Fluorouracil, 5 -FU; Fluorodeoxyuridine; Cytarabine). d) Purine analogs (6 Mercaptopurine, 6 -MP; 6 -Thioguanine, 6 -TG). • 2) Alkylating agents: Nitrogen mustards; Cyclophosphamide; Thiotepa. • 3) Natural products: Vinca alkaloids (Vincristine; Vinblastine; Vinorelbine); Paclitaxel (Taxol® )

• 4) Antiumor antibiotics: Anthracyclines (Doxorubicin hydrochloride, Daunorubicin, Bleomycin) • 5) Miscellaneous agents: Cisplatin; Carboplatin; Asparaginase; Hydroxyurea; Corticosteroid

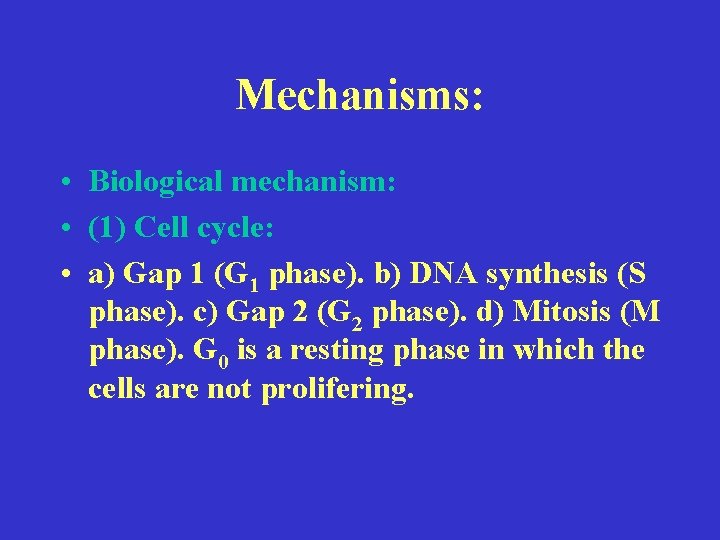
Mechanisms: • Biological mechanism: • (1) Cell cycle: • a) Gap 1 (G 1 phase). b) DNA synthesis (S phase). c) Gap 2 (G 2 phase). d) Mitosis (M phase). G 0 is a resting phase in which the cells are not prolifering.


• (2) Cell cycle nonspecific agents (CCNSA): • Kill proliferating cells preferentially, act on cells at all phases. • (3) Cell cycle specific agents (CCSA): • Act at specific phase of the cell cycle.

• Biochemic mechanism: • 1) Interfere nucleotide synthesis. • 2) Impact the structure and function of DNA • 3) Interfere transcription and block RNA synthesis. • 4) Interfere protein synthesis and functions. • 5) Change hormone lever.

Resistance mechanism • (1) Defective activation: Cyclophosphamide requires metabolic activation, Methotrexate conversion to more active MTXpolyglutamate in cells

• (2) Increased inactivation: e. g. , aldehyde dehydrogenase converse cyclophosphamide to inactive metabolite. • (3) Altered nucleotide pools: Can occur with antimetabolites.

• (4) Altered DNA repair: Repair mechanisms increased, i. e. , ability to remove cross-links, Affect the action of bleomycin and other DNA-directed drugs • (5) Altered target: Less affinity for drug, Methotrexate (Dihydrofolate reductase changes ).

• (6) Decreased target: decreased topoisomerase II, e. g. , etoposide • (7) Gene amplification: Methotrexate (MTX) increase dihydrofolate reductase, hence Requires more MTX to block • (8) Decreased accumulation: Decreased uptake (Methotrexate -- carrier protein decreases). Increased Efflux (Multidrug Resistance, P-Glycoprotein (g. P-170) in membrane, pumps drug out)

Commonly used antineoplastic drugs • Antimetabolites • Group Characteristics: • (1) Resemble NORMAL substrates. • (2) Most inhibit DNA synthesis. • (3) Some inhibit RNA synthesis and/or function. (4) Bone Marrow cell replication is profoundly inhibited. • (5) GI toxicity great with some drugs. • (6) Highly cell cycle specific, also "phase specific", e. g. , S or M phase

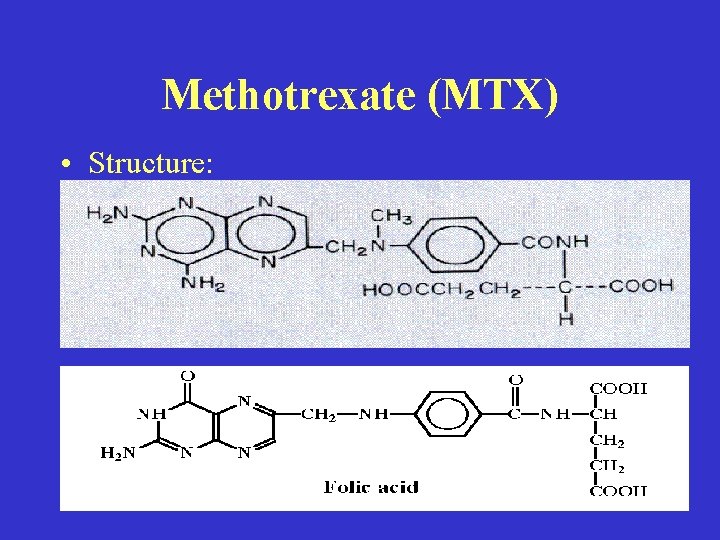
Methotrexate (MTX) • Structure:

• Mechanism of action: • (1) Folic Acid Analogue, Carrier transport into cell. (2) Binds strongly to DHFR to deplete THF, Decreases 1 carbon transfers in Purine synthesis, Decreases [1 -C-THF] intracellular which decreases d. UMP d. TMP, Therefore, decreases NUCLEIC ACID synthesis.

• Adverse effects: • (1) Dose limiting: a) Myelosuppression (Thrombocytopenia and Leukopenia, Nadirs 7 -10 days after Rx, Recovery 1421 days). b) GI toxicity (Oral mucositis is early sign of GI toxicity, Severe mucositis, Small bowel ulceration & bleeding, Diarrhea -- requires cessation to prevent perforation of gut )

• (2) Nephrotoxicity: Conventional doses, infrequent toxicity; High doses, toxicity can be severe • (3) Immunosuppression. • (4) Hepatotoxicity.

• Clinical Uses: • Broad range. Well established: (1) Acute Lymphoblastic Leukemia of childhood. (2) Choriocarcinoma. (3) Cancers of breast, bladder, and head & neck. (4) Useful in non-Hodgkin's lymphomas

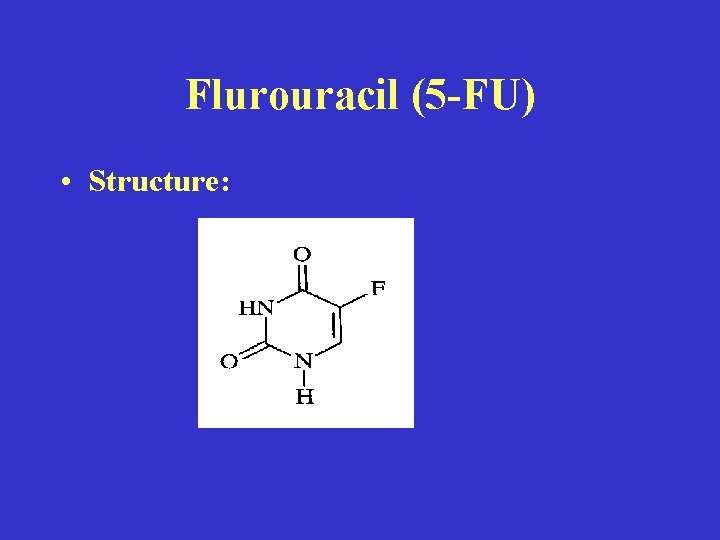
Flurouracil (5 -FU) • Structure:

• Mechanism of action: • (1) Activated by conversion to nucleotide • (2) Inhibits DNA synthesis: Inhibition of Thymidylate synthase—the most important mechanism of action (MOA) in rapidly growing tumors (? ) • (3) 5 -FU Incorporated into RNA: Interfere with RNA processing - All types, May be most important MOA in slowly growing tumors.

• Adverse effects: • (1) Dose limiting: a) Bone marrow -- esp. with bolus administration. Leukopenia & Thrombocytopenia (nadir 9 -14 days after 5 days of Rx, recovery by day 21). b) GI Toxicity -- esp. with infusion administration. usually Stomatitis & Diarrhea 4 -7 days after Rx.

• (2) Effect of route and schedule on adverse effects: • IV bolus: myelosuppression is dominant; Prolonged Rx, may cause megaloblastic anemia • Continuous IV Infusion: Frequently produce, stomatitis, nausea, vomiting, and diarrhea; Hepatotoxicity (elevated transaminases); myelosuppression less common

• (3) Effect of peak 5 -FU concentration: • Acute, reversible cerebellar syndrome: somnolence, ataxia of trunk or extremities, unsteady gait, slurred speech, nystagmus

• (4) Other adverse effects: • Hyperpigmentation of skin is frequent and may be accompanied by photosensitivity; Toxic effect of radiation to skin may be enhanced; Alopecia, acute and chronic conjunctivitis, and nail changes may be observed.

• Clinical Use of 5 -FU: • (1) Single agent: Palliative in advanced colorectal carcinoma • (2) Combination: Breast cancer; Carcinomas of ovary, stomach, pancreas • (3) Sequential MTX + 5 -FU: Head and neck cancer

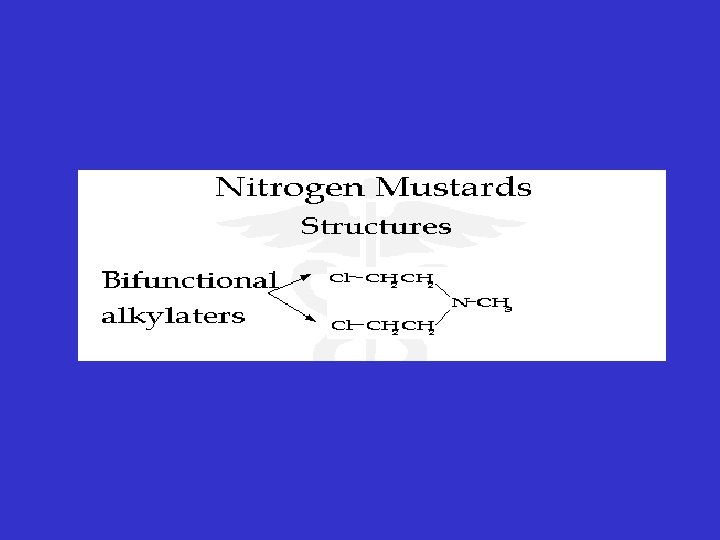
Alkylating Agents • Nitrogen mustard • General view: • (1) Developed from mustard war gases of Word War I which were highly reactive vesicants. • (2) First chemicals used for cancer Rx. • (3) Not cell cycle specific, but still more active in dividing tissues. • (4)"Radiomimetic" -- action on DNA resembles radiation.


• Mechanism of action: • (1) Highly reactive: Form covalent bonds with NDA, RNA and protein • (2) Consequences: a) DNA-DNA strand DNA-Protein cross-links. b) Misreading of genetic code. c) DNA Chain breaks

• Adverse effects of alkylating agents: • (1) More toxic to bone marrow and gut than to liver and kidney, etc. (2) Infertility to both males and females. (3) Mutagenic. (4) Carcinogenic. • Tumor resistance: • Develops slowly & may require several genetic / biochemical changes

• Clinical Uses: • Wide spectrum; Lymphoreticular tissue tumors; Limited activity against sarcomas.

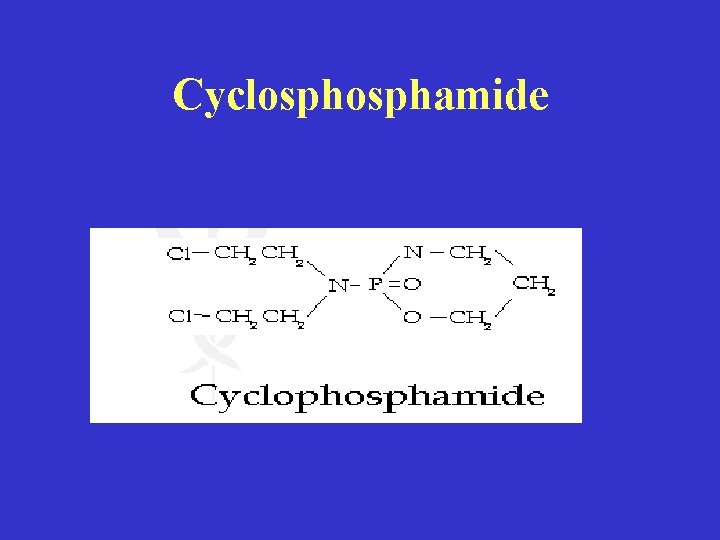
Cyclosphamide

• Mechanism of action: • Hepatic cytochrome P-450 system, enzymes phosphatase and phosphamidase are primary activators (hydrolyze P-N bond) to intermediate, aldophosphamide, which nonenzymatically breaks down to -Phosphoramide mustard (bifunctional) & Acrolein

• Pharmacokinetics: • Oral bioavailability = 90 -100%, IV injection no local irritation • Half-life -- cyclophosphamide -- 3 -10 h; aldophosphamide -1. 6 h; phosphoramide mustard -- 8. 7 h. • Most metabolized-- < 14% unchanged in urine.

• Clinical Applications: • (1) Most widely used alkylating agent, in part due to availability of oral route • (2) Active on lymphoproliferative diseases, e. g. , Hodgkin's disease and Chronic lymphocytic leukemia

• (3) Significant activity vs multiple myeloma & ovarian, breast, small cell lung carcinoma • (4) Many combinations.

• Adverse effects: • (1) Bone marrow suppression, most important leukopenia and thrombocytopenia • (1) Nausea and vomiting said to be rare • (3) Sterile necrotizing hemorrhagic cystitis. Acrolein is probable cause. To minimize cyctitis--high water intake and take in AM

Natural Products • Vinca Alkaloids • Vincristine sulfate and Vinblastine sulfate

• Mechanism of action: • (1) Uptake by energy dependent carrier • (2) Bind to tubulin in microtubules to cause their dissolution. Contrast to Taxol which stabilizes tubules. • (3) No cross resistance between vincristine and vinblastine

• Uses: • (1) Drug of choice for childhood leukemias in combination with prednisone • (2) Used for lymphoreticular neoplasms, carcinomas, and sarcomas

• Adverse effects: • (1) Severe vesicant. Must be careful of IV equipment to avoid slough. • (2) Neurotoxicity: a) Mild sensory neuropathy with sensory impairment and paresthesia--Keep Rx. b) Severe paresthesias, loss of reflexes, ataxia, and muscle wasting-- stop Rx. c) Constipation and abdominal pain - take laxatives. e) Less hematologic effects than many other cytotoxic drugs.

Antitumor Antibiotics • General characteristics: • (1) All interact with DNA and/or RNA, but may also interact with other cellular substituents. • (2) Schedule dependence: LESS "phasespecific" than antimetabolites. • (3) Tissue necrosis is only generalizable toxicity. • (4)All IV except bleomycin

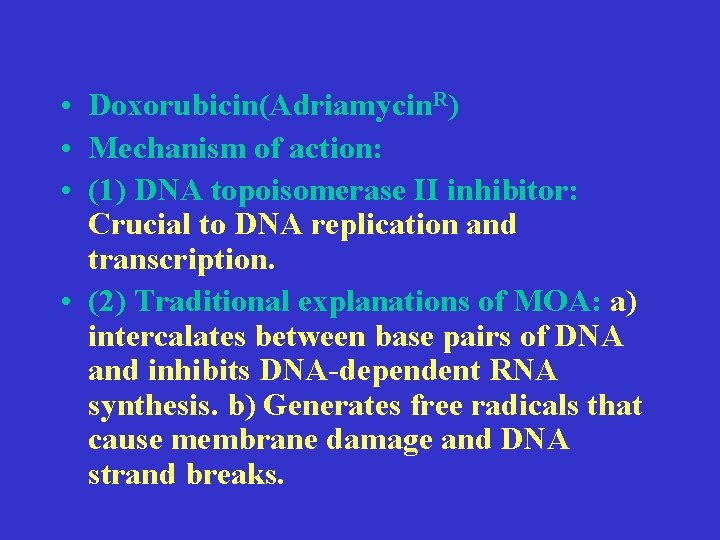
• Doxorubicin(Adriamycin. R) • Mechanism of action: • (1) DNA topoisomerase II inhibitor: Crucial to DNA replication and transcription. • (2) Traditional explanations of MOA: a) intercalates between base pairs of DNA and inhibits DNA-dependent RNA synthesis. b) Generates free radicals that cause membrane damage and DNA strand breaks.

• Resistance: • (1) Alterations in Topoisomerase II activity. • (2) Increased inactivation of radicals: a) Increase in glutathione-dependent enzymes, e. g. , glutathione-peroxidase. b) Altered NADPH contents. • (3) Increase drug efflux: a) Multi-drug resistance (MDR). b) P-glycoprotein (g. P 170) pump is product of mdr gene

• Adverse effects: • Three categories of toxicity: a) Local toxicities. b) Acute toxicities. c) Chronic toxicity • (1) Local Toxicity -- Extravasation • Extravasation -- DON'T! Severe local tissue necrosis to point of damaging underlying structures; If occurs, treat immediately: Remove blood from IV line; Apply ice, steroid cream; Locally adm. sodium bicarbonate and hydrocortisone.

• (2) Local Toxicity -- Radiation Recall • Interaction of doxorubicin and radiation in some tissues to produce enhanced reactions. • Reactions include: a) Skin: ulceration and necrosis. b) Pulmonary: fibrosis and sloughing of esophageal mucosa. c) Heart, and intestinal mucosa may also be affected

• (3) Acute Toxicities • a) Hematologic: Leukopenia with nadir 710 days; recovery typically by 21 days; Thrombocytopenia and anemia less common • b) If give too fast: "Histamine-release" syndrome; Cardiac arrest preceded by ECG changes

• (4) Chronic Toxicities • a) Cardiomyopathy and congestive heart failure: require cessation of Rx after cumulative dose of 550 to 600 mg/m 2; must maintain record of total dose.

• Clinical Indications: • (1) Broad spectrum anti-cancer activity. • (2) Hodgkin's disease, non-Hodgkin's lymphomas, sarcomas, acute leukemia, and breast, lung, and ovarian carcinomas all responsive • (3) Activity observed in bladder tumors, and carcinomas of prostate, thyroid, endometrium, head and neck, and other solid tumors • (The end)
 Pharmacology of drugs acting on respiratory system
Pharmacology of drugs acting on respiratory system Adrenal drugs pharmacology
Adrenal drugs pharmacology Alex liu cecilia liu
Alex liu cecilia liu Líu líu lo lo ta ca hát say sưa
Líu líu lo lo ta ca hát say sưa Chapter 30 principles of pharmacology
Chapter 30 principles of pharmacology Chapter 15 diagnostic procedures and pharmacology
Chapter 15 diagnostic procedures and pharmacology Pharmacology chapter 1
Pharmacology chapter 1 Medical education and drugs department
Medical education and drugs department Venipuncture radiologic technologist
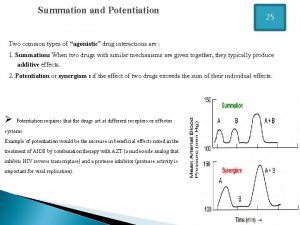
Venipuncture radiologic technologist Potentiation pharmacology example
Potentiation pharmacology example Creatinine clearance formula
Creatinine clearance formula What is ion trapping in pharmacology
What is ion trapping in pharmacology Hagar conjugation
Hagar conjugation First pass metabolism
First pass metabolism Alia drug testing
Alia drug testing First pass effect in pharmacology
First pass effect in pharmacology First pass effect in pharmacology
First pass effect in pharmacology Mechanism of drug action
Mechanism of drug action First pass effect
First pass effect Alpha 1 vs alpha 2 receptors
Alpha 1 vs alpha 2 receptors What is pharmacology
What is pharmacology Slidetodoc
Slidetodoc Competitive and non competitive antagonist
Competitive and non competitive antagonist What is ion trapping in pharmacology
What is ion trapping in pharmacology Basic principles of pharmacology
Basic principles of pharmacology Pharmacology for nurses: a pathophysiological approach
Pharmacology for nurses: a pathophysiological approach Respiratory pharmacology quiz
Respiratory pharmacology quiz Pharmacology module
Pharmacology module Hepatic extraction ratio formula
Hepatic extraction ratio formula Clinical pharmacology powered by clinicalkey
Clinical pharmacology powered by clinicalkey Pharmacology pay
Pharmacology pay Pharmacology definition
Pharmacology definition Toxicology and applied pharmacology
Toxicology and applied pharmacology Rationale meaning in pharmacology
Rationale meaning in pharmacology Pharmacology tutor anderson
Pharmacology tutor anderson Clinical pharmacology
Clinical pharmacology Basic & clinical pharmacology
Basic & clinical pharmacology Dopamine synthesis
Dopamine synthesis Branches of pharmacology
Branches of pharmacology Pharmacology for veterinary technicians
Pharmacology for veterinary technicians Chronic gout
Chronic gout Clinical pharmacology residency
Clinical pharmacology residency Annual review of pharmacology and toxicology
Annual review of pharmacology and toxicology Dopamine blockers
Dopamine blockers Define pharmacology
Define pharmacology Concept of essential drugs
Concept of essential drugs Samyukti meaning
Samyukti meaning Glomerular
Glomerular