Chapter 40 Introduction to Quantum Physics Need for



















- Slides: 64

Chapter 40 Introduction to Quantum Physics

Need for Quantum Physics Problems remained from classical mechanics that the special theory of relativity didn’t explain. Attempts to apply the laws of classical physics to explain the behavior of matter on the atomic scale were consistently unsuccessful. Problems included: § Blackbody radiation § The electromagnetic radiation emitted by a heated object § Photoelectric effect § Emission of electrons by an illuminated metal Introduction

Quantum Mechanics Revolution Between 1900 and 1930, another revolution took place in physics. A new theory called quantum mechanics was successful in explaining the behavior of particles of microscopic size. The first explanation using quantum theory was introduced by Max Planck. § Many other physicists were involved in other subsequent developments Introduction

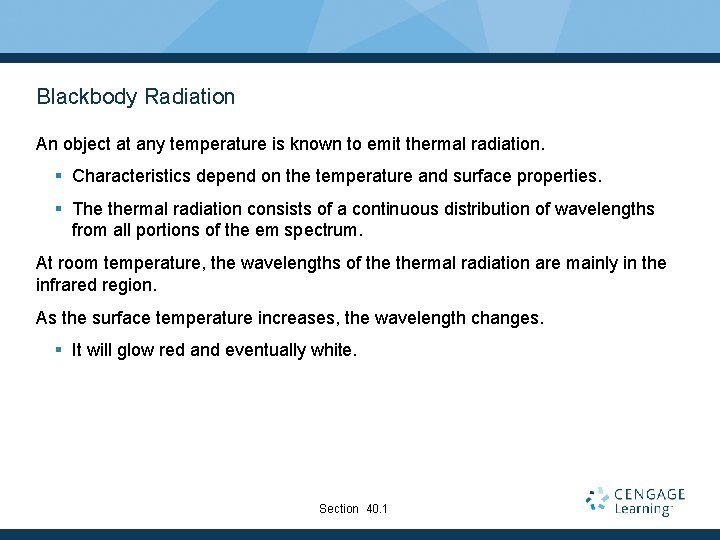
Blackbody Radiation An object at any temperature is known to emit thermal radiation. § Characteristics depend on the temperature and surface properties. § The thermal radiation consists of a continuous distribution of wavelengths from all portions of the em spectrum. At room temperature, the wavelengths of thermal radiation are mainly in the infrared region. As the surface temperature increases, the wavelength changes. § It will glow red and eventually white. Section 40. 1

Blackbody Radiation, cont. The basic problem was in understanding the observed distribution in the radiation emitted by a black body. § Classical physics didn’t adequately describe the observed distribution. A black body is an ideal system that absorbs all radiation incident on it. The electromagnetic radiation emitted by a black body is called blackbody radiation. Section 40. 1

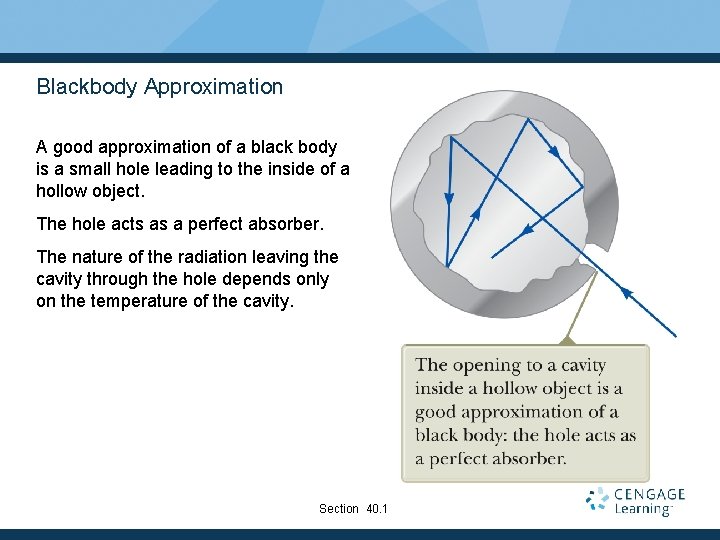
Blackbody Approximation A good approximation of a black body is a small hole leading to the inside of a hollow object. The hole acts as a perfect absorber. The nature of the radiation leaving the cavity through the hole depends only on the temperature of the cavity. Section 40. 1

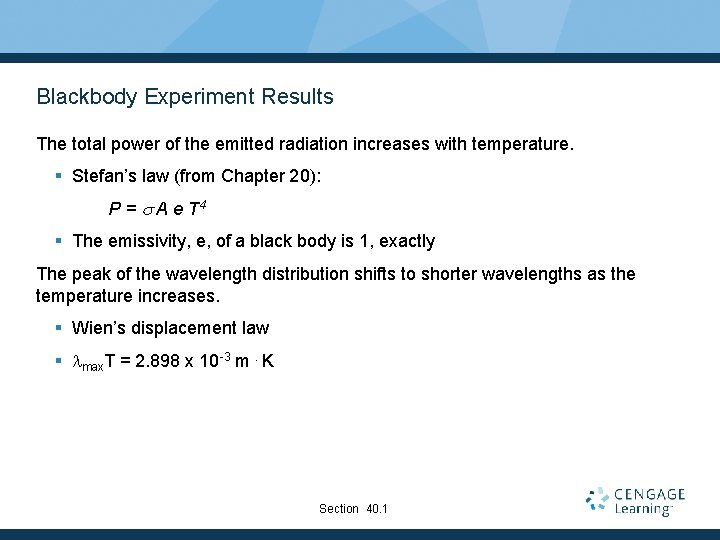
Blackbody Experiment Results The total power of the emitted radiation increases with temperature. § Stefan’s law (from Chapter 20): P = s A e T 4 § The emissivity, e, of a black body is 1, exactly The peak of the wavelength distribution shifts to shorter wavelengths as the temperature increases. § Wien’s displacement law § lmax. T = 2. 898 x 10 -3 m. K Section 40. 1

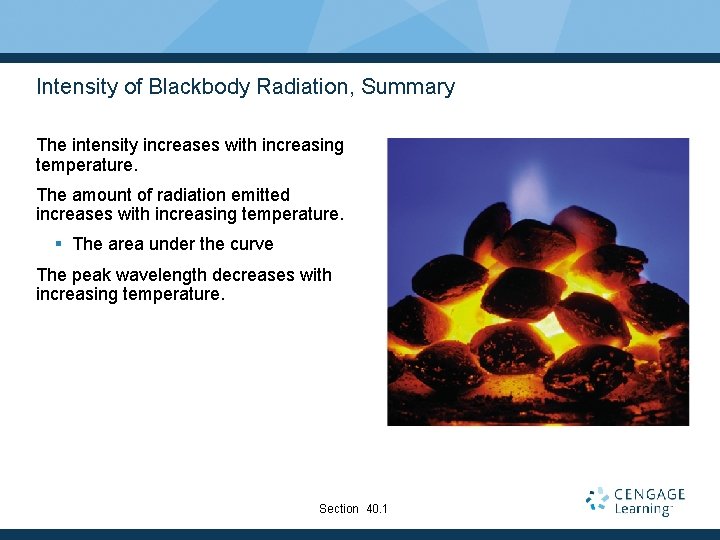
Intensity of Blackbody Radiation, Summary The intensity increases with increasing temperature. The amount of radiation emitted increases with increasing temperature. § The area under the curve The peak wavelength decreases with increasing temperature. Section 40. 1

Rayleigh-Jeans Law An early classical attempt to explain blackbody radiation was the Rayleigh. Jeans law. At long wavelengths, the law matched experimental results fairly well. Section 40. 1

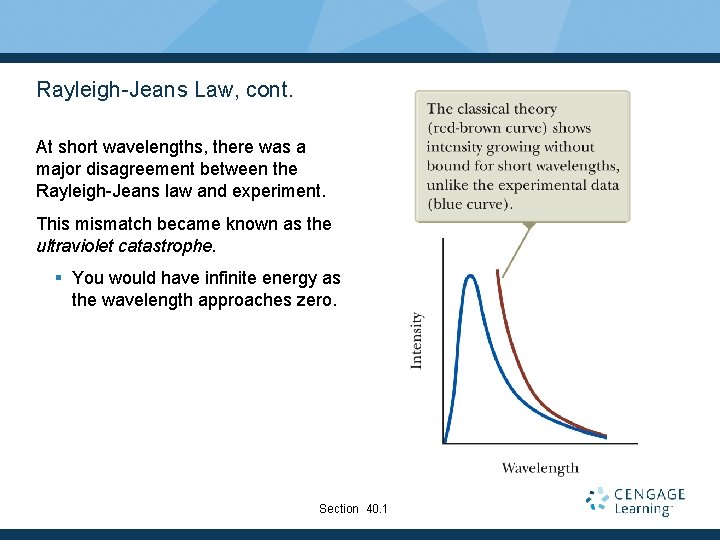
Rayleigh-Jeans Law, cont. At short wavelengths, there was a major disagreement between the Rayleigh-Jeans law and experiment. This mismatch became known as the ultraviolet catastrophe. § You would have infinite energy as the wavelength approaches zero. Section 40. 1

Max Planck 1858 – 1847 German physicist Introduced the concept of “quantum of action” In 1918 he was awarded the Nobel Prize for the discovery of the quantized nature of energy. Section 40. 1

Planck’s Theory of Blackbody Radiation In 1900 Planck developed a theory of blackbody radiation that leads to an equation for the intensity of the radiation. This equation is in complete agreement with experimental observations. He assumed the cavity radiation came from atomic oscillations in the cavity walls. Planck made two assumptions about the nature of the oscillators in the cavity walls. Section 40. 1

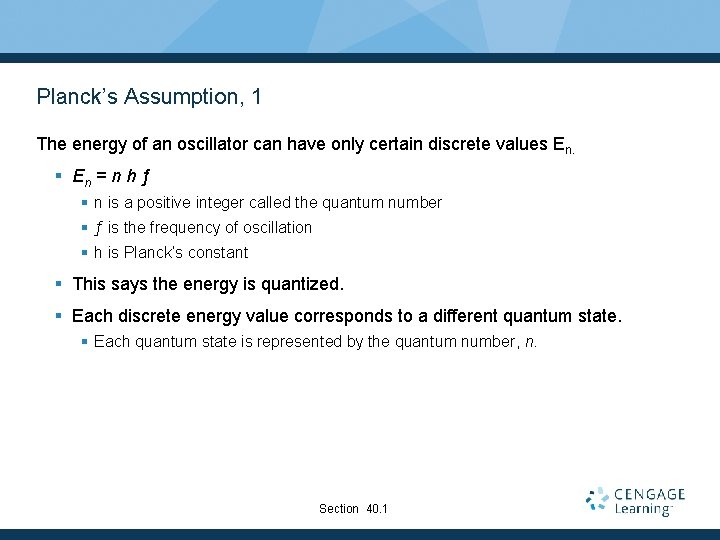
Planck’s Assumption, 1 The energy of an oscillator can have only certain discrete values En. § En = n h ƒ § n is a positive integer called the quantum number § ƒ is the frequency of oscillation § h is Planck’s constant § This says the energy is quantized. § Each discrete energy value corresponds to a different quantum state. § Each quantum state is represented by the quantum number, n. Section 40. 1

Planck’s Assumption, 2 The oscillators emit or absorb energy when making a transition from one quantum state to another. § The entire energy difference between the initial and final states in the transition is emitted or absorbed as a single quantum of radiation. § An oscillator emits or absorbs energy only when it changes quantum states. § The energy carried by the quantum of radiation is E = h ƒ. Section 40. 1

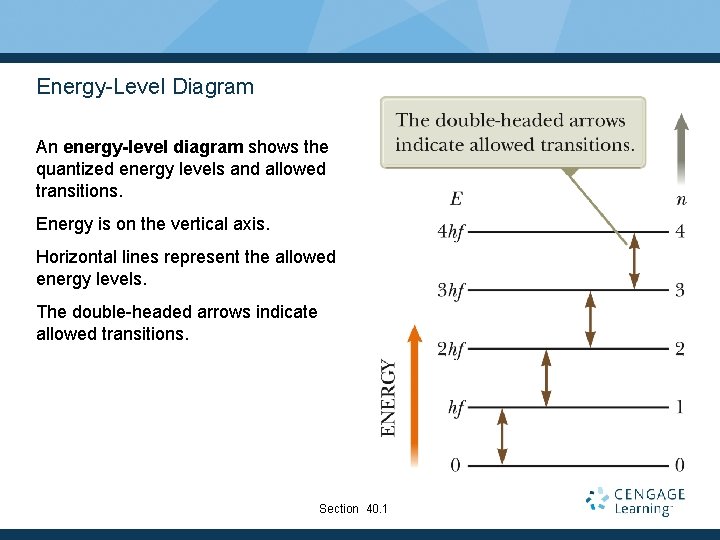
Energy-Level Diagram An energy-level diagram shows the quantized energy levels and allowed transitions. Energy is on the vertical axis. Horizontal lines represent the allowed energy levels. The double-headed arrows indicate allowed transitions. Section 40. 1

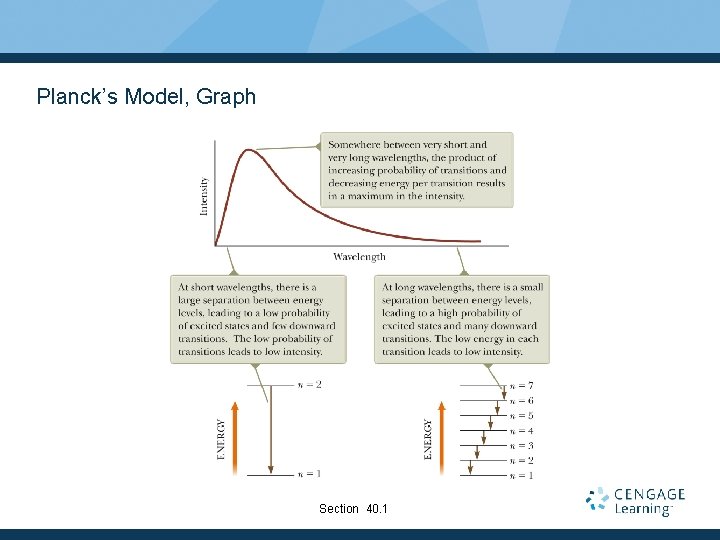
More About Planck’s Model The average energy of a wave is the average energy difference between levels of the oscillator, weighted according to the probability of the wave being emitted. This weighting is described by the Boltzmann distribution law and gives the probability of a state being occupied as being proportional to where E is the energy of the state. Section 40. 1

Planck’s Model, Graph Section 40. 1

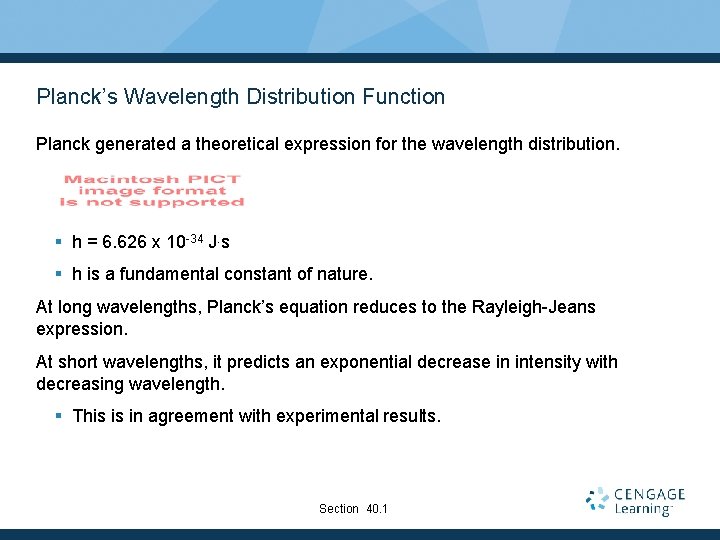
Planck’s Wavelength Distribution Function Planck generated a theoretical expression for the wavelength distribution. § h = 6. 626 x 10 -34 J. s § h is a fundamental constant of nature. At long wavelengths, Planck’s equation reduces to the Rayleigh-Jeans expression. At short wavelengths, it predicts an exponential decrease in intensity with decreasing wavelength. § This is in agreement with experimental results. Section 40. 1

Einstein and Planck’s Results Einstein rederived Planck’s results by assuming the oscillations of the electromagnetic field were themselves quantized. In other words, Einstein proposed that quantization is a fundamental property of light and other electromagnetic radiation. This led to the concept of photons. Section 40. 1

Photoelectric Effect The photoelectric effect occurs when light incident on certain metallic surfaces causes electrons to be emitted from those surfaces. § The emitted electrons are called photoelectrons. § They are no different than other electrons. § The name is given because of their ejection from a metal by light in the photoelectric effect Section 40. 2

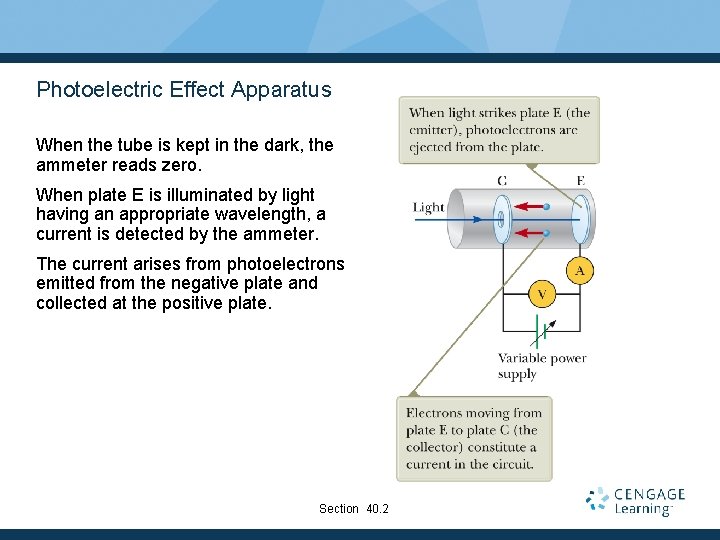
Photoelectric Effect Apparatus When the tube is kept in the dark, the ammeter reads zero. When plate E is illuminated by light having an appropriate wavelength, a current is detected by the ammeter. The current arises from photoelectrons emitted from the negative plate and collected at the positive plate. Section 40. 2

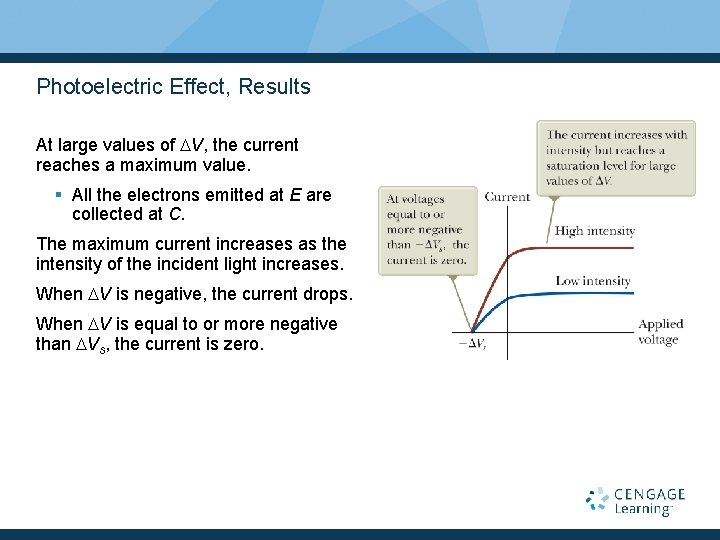
Photoelectric Effect, Results At large values of DV, the current reaches a maximum value. § All the electrons emitted at E are collected at C. The maximum current increases as the intensity of the incident light increases. When DV is negative, the current drops. When DV is equal to or more negative than DVs, the current is zero.

Photoelectric Effect Feature 1 Dependence of photoelectron kinetic energy on light intensity § Classical Prediction § Electrons should absorb energy continually from the electromagnetic waves. § As the light intensity incident on the metal is increased, the electrons should be ejected with more kinetic energy. § Experimental Result § The maximum kinetic energy is independent of light intensity. § The maximum kinetic energy is proportional to the stopping potential (DVs). Section 40. 2

Photoelectric Effect Feature 2 Time interval between incidence of light and ejection of photoelectrons § Classical Prediction § At low light intensities, a measurable time interval should pass between the instant the light is turned on and the time an electron is ejected from the metal. § This time interval is required for the electron to absorb the incident radiation before it acquires enough energy to escape from the metal. § Experimental Result § Electrons are emitted almost instantaneously, even at very low light intensities. Section 40. 2

Photoelectric Effect Feature 3 Dependence of ejection of electrons on light frequency § Classical Prediction § Electrons should be ejected at any frequency as long as the light intensity is high enough. § Experimental Result § No electrons are emitted if the incident light falls below some cutoff frequency, ƒc. § The cutoff frequency is characteristic of the material being illuminated. § No electrons are ejected below the cutoff frequency regardless of intensity. Section 40. 2

Photoelectric Effect Feature 4 Dependence of photoelectron kinetic energy on light frequency § Classical Prediction § There should be no relationship between the frequency of the light and the electric kinetic energy. § The kinetic energy should be related to the intensity of the light. § Experimental Result § The maximum kinetic energy of the photoelectrons increases with increasing light frequency. Section 40. 2

Photoelectric Effect Features, Summary The experimental results contradict all four classical predictions. Einstein extended Planck’s concept of quantization to electromagnetic waves. All electromagnetic radiation of frequency ƒ from any source can be considered a stream of quanta, now called photons. Each photon has an energy E and moves at the speed of light in a vacuum. § E = hƒ A photon of incident light gives all its energy to a single electron in the metal. Section 40. 2

Photoelectric Effect, Work Function Electrons ejected from the surface of the metal and not making collisions with other metal atoms before escaping possess the maximum kinetic energy Kmax = hƒ – φ § φ is called the work function of the metal. § The work function represents the minimum energy with which an electron is bound in the metal. Section 40. 2

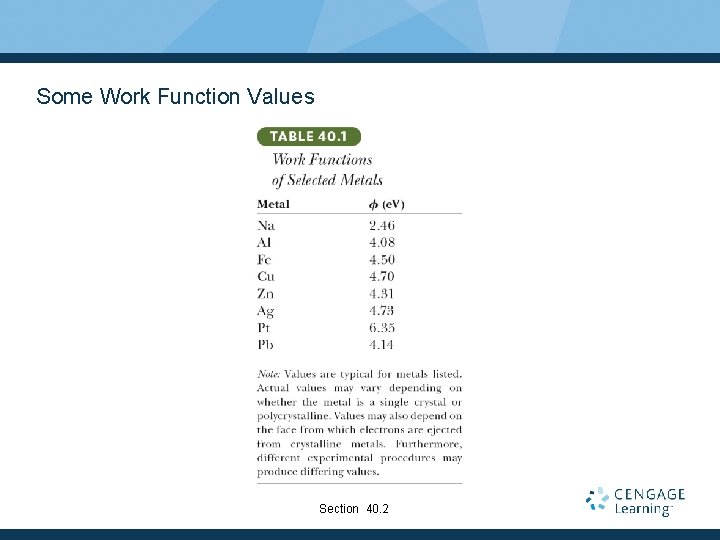
Some Work Function Values Section 40. 2

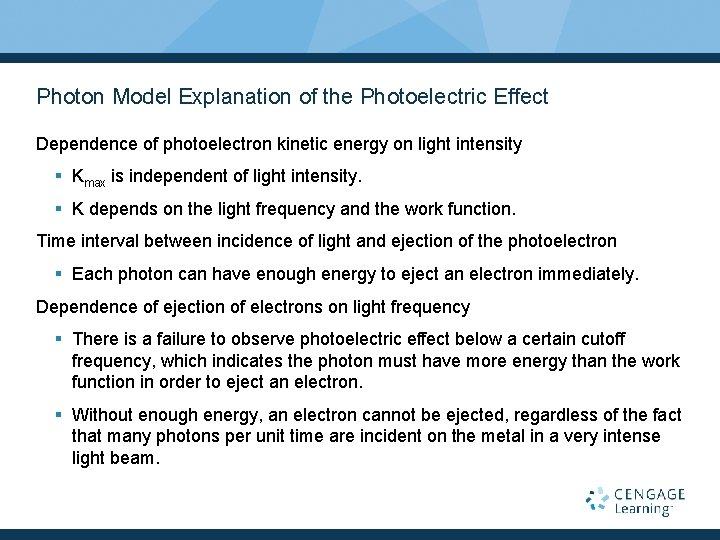
Photon Model Explanation of the Photoelectric Effect Dependence of photoelectron kinetic energy on light intensity § Kmax is independent of light intensity. § K depends on the light frequency and the work function. Time interval between incidence of light and ejection of the photoelectron § Each photon can have enough energy to eject an electron immediately. Dependence of ejection of electrons on light frequency § There is a failure to observe photoelectric effect below a certain cutoff frequency, which indicates the photon must have more energy than the work function in order to eject an electron. § Without enough energy, an electron cannot be ejected, regardless of the fact that many photons per unit time are incident on the metal in a very intense light beam.

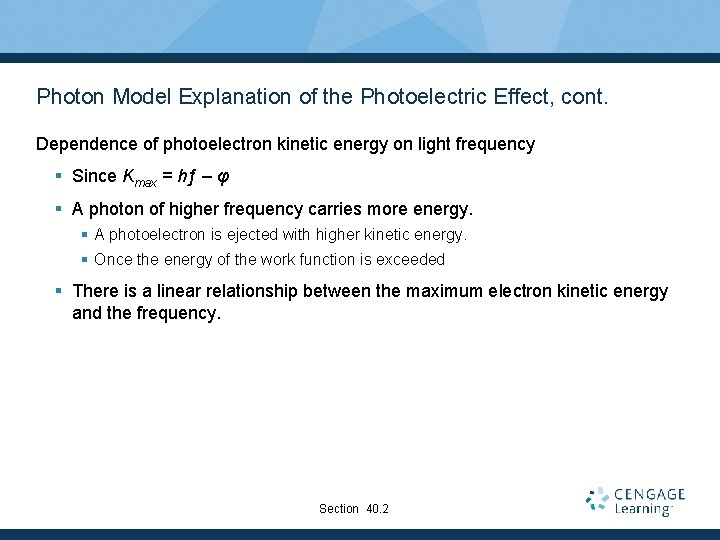
Photon Model Explanation of the Photoelectric Effect, cont. Dependence of photoelectron kinetic energy on light frequency § Since Kmax = hƒ – φ § A photon of higher frequency carries more energy. § A photoelectron is ejected with higher kinetic energy. § Once the energy of the work function is exceeded § There is a linear relationship between the maximum electron kinetic energy and the frequency. Section 40. 2

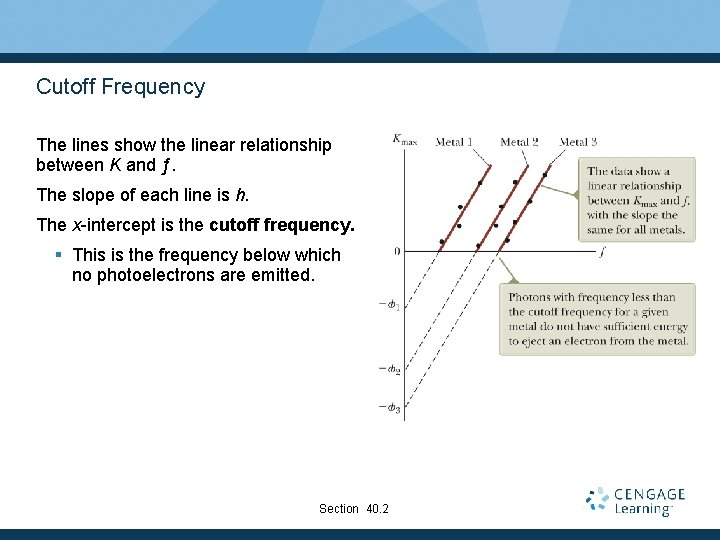
Cutoff Frequency The lines show the linear relationship between K and ƒ. The slope of each line is h. The x-intercept is the cutoff frequency. § This is the frequency below which no photoelectrons are emitted. Section 40. 2

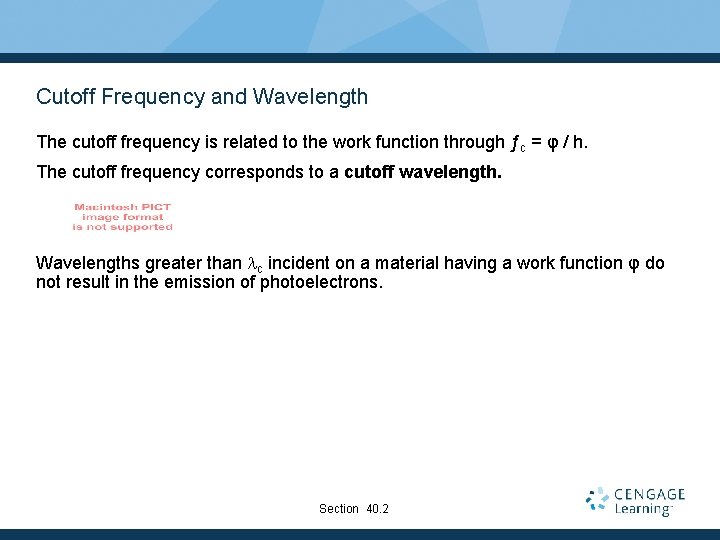
Cutoff Frequency and Wavelength The cutoff frequency is related to the work function through ƒc = φ / h. The cutoff frequency corresponds to a cutoff wavelength. Wavelengths greater than lc incident on a material having a work function φ do not result in the emission of photoelectrons. Section 40. 2

Arthur Holly Compton 1892 – 1962 American physicist Director of the lab at the University of Chicago Discovered the Compton Effect Shared the Nobel Prize in 1927 Section 40. 3

The Compton Effect, Introduction Compton and Debye extended Einstein’s idea of photon momentum. The two groups of experimenters accumulated evidence of the inadequacy of the classical wave theory. The classical wave theory of light failed to explain the scattering of x-rays from electrons. Section 40. 3

Compton Effect, Classical Predictions According to the classical theory, em waves incident on electrons should: § Have radiation pressure that should cause the electrons to accelerate § Set the electrons oscillating § There should be a range of frequencies for the scattered electrons. Section 40. 3

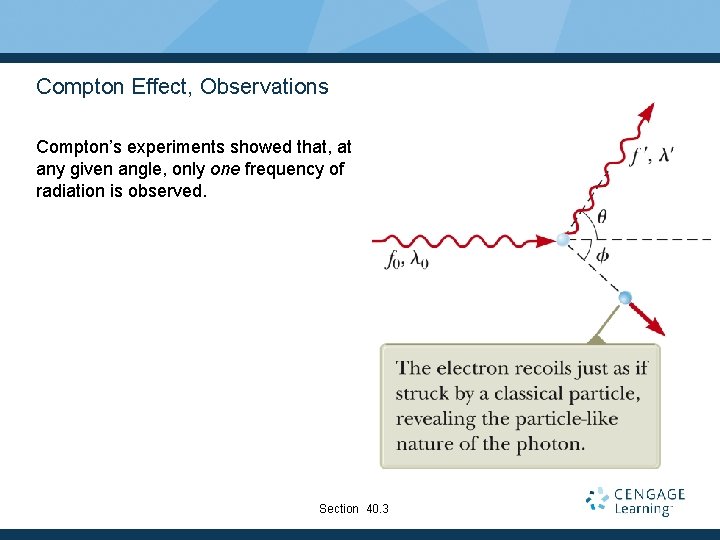
Compton Effect, Observations Compton’s experiments showed that, at any given angle, only one frequency of radiation is observed. Section 40. 3

Compton Effect, Explanation The results could be explained by treating the photons as point-like particles having energy hƒ and momentum h ƒ / c. Assume the energy and momentum of the isolated system of the colliding photon -electron are conserved. This scattering phenomena is known as the Compton effect. Section 40. 3

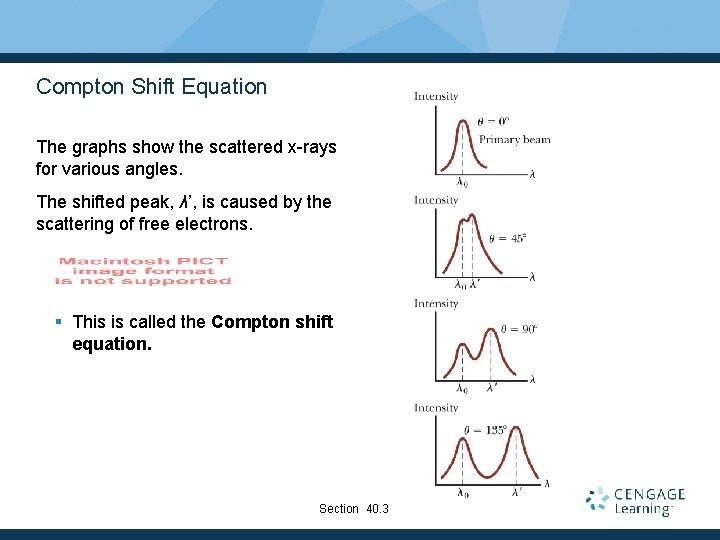
Compton Shift Equation The graphs show the scattered x-rays for various angles. The shifted peak, λ’, is caused by the scattering of free electrons. § This is called the Compton shift equation. Section 40. 3

Compton Wavelength The factor h/mec in the equation is called the Compton wavelength of the electron and is The unshifted wavelength, λo, is caused by x-rays scattered from the electrons that are tightly bound to the target atoms. Section 40. 3

Photons and Waves Revisited Some experiments are best explained by the photon model. Some are best explained by the wave model. We must accept both models and admit that the true nature of light is not describable in terms of any single classical model. The particle model and the wave model of light complement each other. A complete understanding of the observed behavior of light can be attained only if the two models are combined in a complementary matter. Section 40. 4

Louis de Broglie 1892 – 1987 French physicist Originally studied history Was awarded the Nobel Prize in 1929 for his prediction of the wave nature of electrons Section 40. 5

Wave Properties of Particles Louis de Broglie postulated that because photons have both wave and particle characteristics, perhaps all forms of matter have both properties. The de Broglie wavelength of a particle is Section 40. 5

Frequency of a Particle In an analogy with photons, de Broglie postulated that a particle would also have a frequency associated with it These equations present the dual nature of matter: § Particle nature, p and E § Wave nature, λ and ƒ Section 40. 5

Complementarity The principle of complementarity states that the wave and particle models of either matter or radiation complement each other. Neither model can be used exclusively to describe matter or radiation adequately. Section 40. 5

Davisson-Germer Experiment If particles have a wave nature, then under the correct conditions, they should exhibit diffraction effects. Davisson and Germer measured the wavelength of electrons. This provided experimental confirmation of the matter waves proposed by de Broglie. Section 40. 5

Wave Properties of Particles Mechanical waves have materials that are “waving” and can be described in terms of physical variables. § A string may be vibrating. § Sound waves are produced by molecules of a material vibrating. § Electromagnetic waves are associated with electric and magnetic fields. Waves associated with particles cannot be associated with a physical variable. Section 40. 5

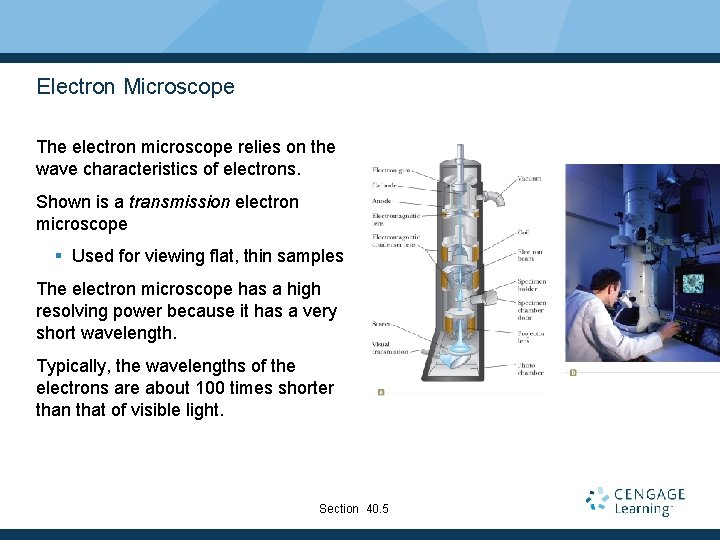
Electron Microscope The electron microscope relies on the wave characteristics of electrons. Shown is a transmission electron microscope § Used for viewing flat, thin samples The electron microscope has a high resolving power because it has a very short wavelength. Typically, the wavelengths of the electrons are about 100 times shorter than that of visible light. Section 40. 5

Quantum Particle The quantum particle is a new model that is a result of the recognition of the dual nature of both light and material particles. Entities have both particle and wave characteristics. We must choose one appropriate behavior in order to understand a particular phenomenon. Section 40. 6

Ideal Particle vs. Ideal Wave An ideal particle has zero size. § Therefore, it is localized in space. An ideal wave has a single frequency and is infinitely long. § Therefore, it is unlocalized in space. A localized entity can be built from infinitely long waves. Section 40. 6

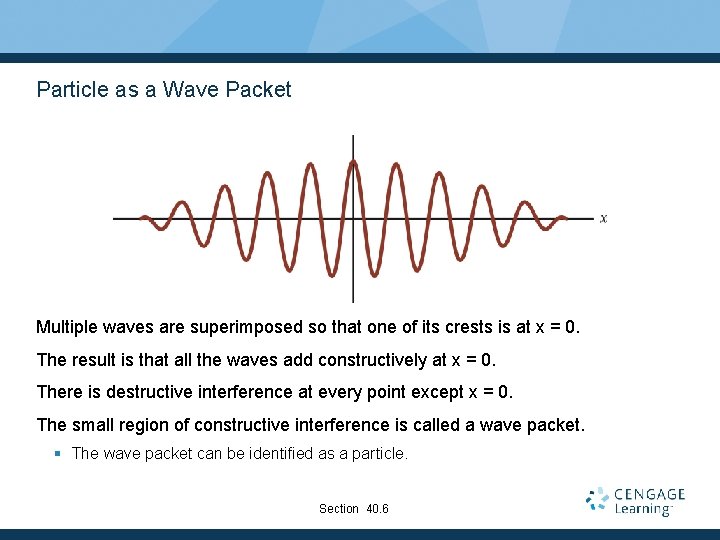
Particle as a Wave Packet Multiple waves are superimposed so that one of its crests is at x = 0. The result is that all the waves add constructively at x = 0. There is destructive interference at every point except x = 0. The small region of constructive interference is called a wave packet. § The wave packet can be identified as a particle. Section 40. 6

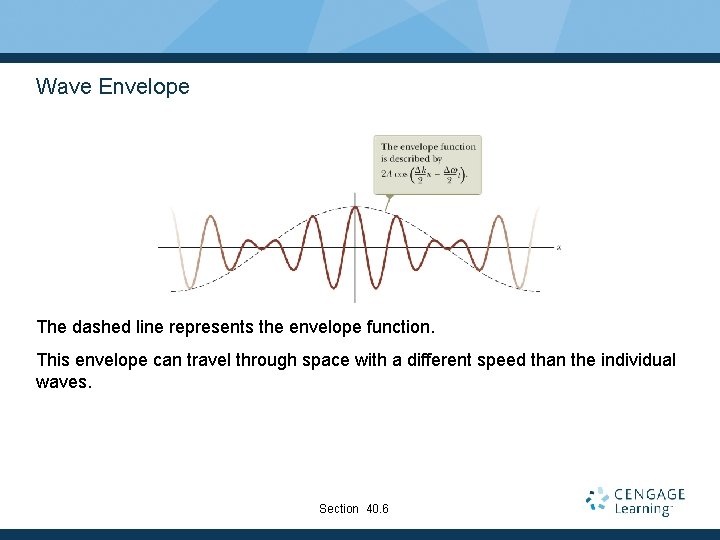
Wave Envelope The dashed line represents the envelope function. This envelope can travel through space with a different speed than the individual waves. Section 40. 6

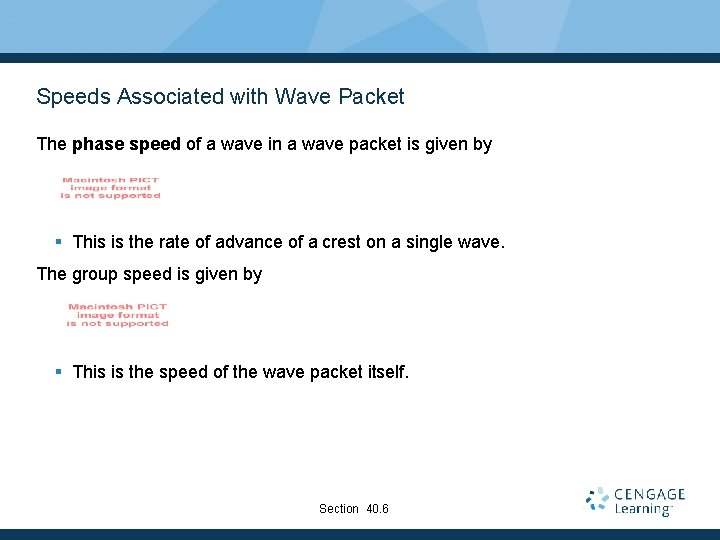
Speeds Associated with Wave Packet The phase speed of a wave in a wave packet is given by § This is the rate of advance of a crest on a single wave. The group speed is given by § This is the speed of the wave packet itself. Section 40. 6

Speeds, cont. The group speed can also be expressed in terms of energy and momentum. This indicates that the group speed of the wave packet is identical to the speed of the particle that it is modeled to represent. Section 40. 6

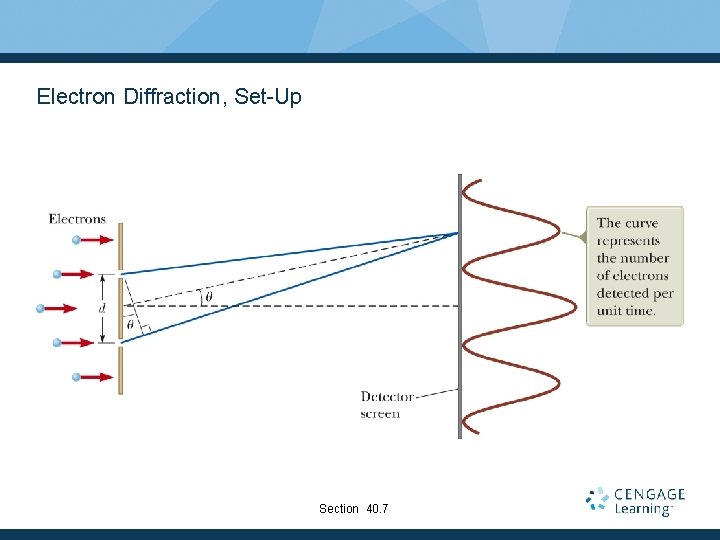
Electron Diffraction, Set-Up Section 40. 7

Electron Diffraction, Experiment Parallel beams of mono-energetic electrons that are incident on a double slit. The slit widths are small compared to the electron wavelength. An electron detector is positioned far from the slits at a distance much greater than the slit separation. Section 40. 7

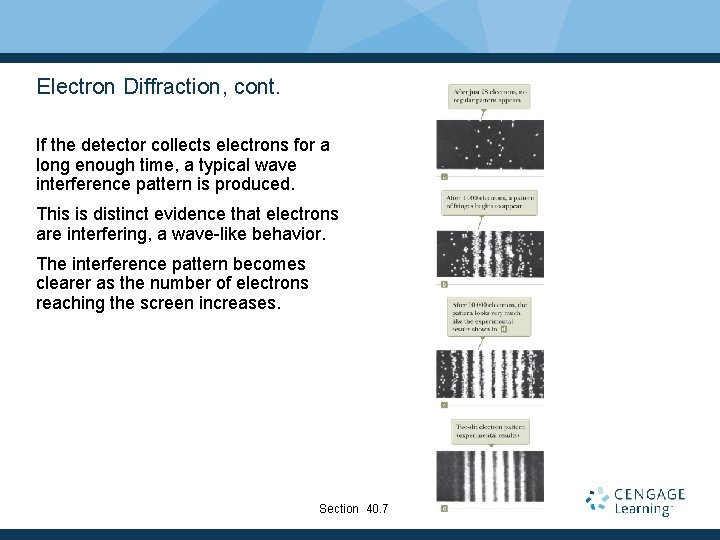
Electron Diffraction, cont. If the detector collects electrons for a long enough time, a typical wave interference pattern is produced. This is distinct evidence that electrons are interfering, a wave-like behavior. The interference pattern becomes clearer as the number of electrons reaching the screen increases. Section 40. 7

Electron Diffraction, Equations A maximum occurs when § This is the same equation that was used for light. This shows the dual nature of the electron. § The electrons are detected as particles at a localized spot at some instant of time. § The probability of arrival at that spot is determined by finding the intensity of two interfering waves. Section 40. 7

Electron Diffraction Explained An electron interacts with both slits simultaneously. If an attempt is made to determine experimentally which slit the electron goes through, the act of measuring destroys the interference pattern. § It is impossible to determine which slit the electron goes through. In effect, the electron goes through both slits. § The wave components of the electron are present at both slits at the same time. Section 40. 7

Werner Heisenberg 1901 – 1976 German physicist Developed matrix mechanics Many contributions include: § Uncertainty principle § Received Nobel Prize in 1932 § Prediction of two forms of molecular hydrogen § Theoretical models of the nucleus Section 40. 8

The Uncertainty Principle In classical mechanics, it is possible, in principle, to make measurements with arbitrarily small uncertainty. Quantum theory predicts that it is fundamentally impossible to make simultaneous measurements of a particle’s position and momentum with infinite accuracy. The Heisenberg uncertainty principle states: if a measurement of the position of a particle is made with uncertainty Dx and a simultaneous measurement of its x component of momentum is made with uncertainty Dpx, the product of the two uncertainties can never be smaller than /2. Section 40. 8

Heisenberg Uncertainty Principle, Explained It is physically impossible to measure simultaneously the exact position and exact momentum of a particle. The inescapable uncertainties do not arise from imperfections in practical measuring instruments. The uncertainties arise from the quantum structure of matter. Section 40. 8

Heisenberg Uncertainty Principle, Another Form Another form of the uncertainty principle can be expressed in terms of energy and time. This suggests that energy conservation can appear to be violated by an amount DE as long as it is only for a short time interval Dt. Section 40. 8

Uncertainty Principle, final The Uncertainty Principle cannot be interpreted as meaning that a measurement interferes with the system. The Uncertainty Principle is independent of the measurement process. It is based on the wave nature of matter. Section 40. 8