Chapter 4 Validation Objectives 1 2 3 4

Chapter 4 Validation

Objectives 1. 2. 3. 4. 5. 6. 7. 8. 9. Define and apply common validation terminology Describe how equipment, process, and method validation fir into the overall quality system Define the types of validation documents found in a biomanufacturing organization and their typical content and purpose Explain the validation lifecycle Describe how risk assessment and analysis are applied to validation activities in the biomanufacturing industry Explain how a validation program is systematically established and the flow of validation requirements involved Distinguish procedures and outcomes for Design Qualification (DQ), Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) Describe the general methods for facility, equipment, and utility validation; analytical method validation; computerized systems validation; process validation; and cleaning validation Summarize the change control and support processes

Validation Exemplifies Process Understanding

Validation Defined by FDA ▪ “The process of demonstrating, through documented evidence, that a process, procedure, piece of equipment, analytical method, or facility will consistently produce a product or result that meets predetermined specifications and quality attributes. ”

Proof of Validation ▪ Documented evidence that § the facility, equipment, and utilities all perform as expected § the analytical methods used in the quality control laboratory perform as expected § each step of the production process contributes to a final product that meets all of the quality attributes and specifications

Validation ▪ Validation is an external check on the performance of a system and ultimately the entire manufacturing process § If the process performs properly, it should produce a product that meets predetermined specifications § If it does not perform properly, a step in the process exists that is either inadequately understood or is not performing as designed
Demonstration and Documentation § Validation also forces the biomanufacturer to examine assumptions about equipment, materials, procedures, and the entire production process one can assume that material placed in an autoclave will be sterilized if the autoclave is working properly § But how can that person know that the autoclave is working properly or that it sterilizes the material to meet the necessary established standard if it is working properly? §

Validation Evolution ▪ Validation remains a time-consuming and expensive process ▪ It is improved from the old mindset validate anything that moves and don’t move anything that is validated ▪ The current approach incorporates process understanding and risk assessment towards an elimination or reduction of those risks
Validation Evolved § Product and process knowledge culminate in validation activities § Documentation that the controlled processes result in products with desired quality attributes § To this end, biomanufacturers should § Understand the sources of variation § Detect the presence and degree of variation § Understand the impact of variation on the process and ultimately on product attributes § Control the variation in a manner commensurate with the risk it represents to the process and product
Viral Inactivation Example Assumptions § Holding the process material at 56 degrees Celsius for 60 minutes will inactivate any and all contaminating viruses. § The container used for the virus inactivation allows all of the material in the container to achieve a temperature of 56 degrees Celsius. § The heating step can be maintained for 60 minutes. § Heating of the product to 56 degrees Celsius for 60 minutes does not impact the quality of the product. Demonstration § § heating a sample to 56 degrees Celsius for 60 minutes will, in fact, inactivate any potential viruses. the entire sample within the container (this might involve thousands of liters at the manufacturing scale) is uniformly heated to 56 degrees Celsius. the heating step can be maintained uniformly throughout the solution for a minimum of 60 minutes. the heating step does not adversely affect the quality of the product (the half-life of typical microbial and viral proteins is approximately two minutes at 55 degrees Celsius).
Demonstrating Process Parameters § Temperature-mapping studies to ensure that all areas within the § § vessel achieve the desired temperature The mixing rate of the material needs to be documented to prove that as the solution is mixed it maintains its temperature. Testing under worse-case scenarios § Maximum volume, lowest mixer setting, partially-operable heater, other plausible mechanical issues § Verification that if specifications are met at the limits of the ranges, the specifications will assuredly be met at the normal operating range § All with no impact on product quality!
Validation Lifecycle § Begins with the design of the manufacturing process § Sustained through the use of equipment and trial production runs § Culminating in a body of evidence that each piece of equipment, each analytical method, each step of the manufacturing process, and each production run will produce a consistent, quality product § Continues through periodic re-validation at specified intervals or in the event of changes § Maintenance of the “validated state”
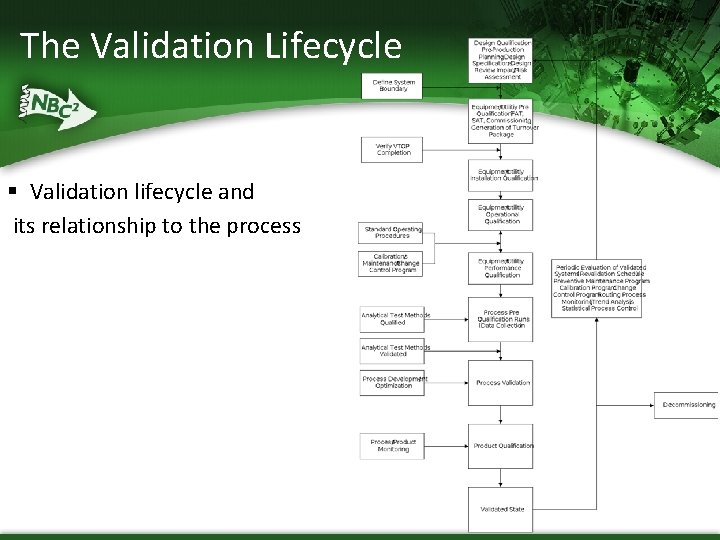
The Validation Lifecycle § Validation lifecycle and its relationship to the process

The Need for Validation § Like many quality requirements, historical accidents and tragedies motivate the need for validation § Cutter Incident § Pseudomonas contamination in large volume parenterals (LVP) § Common theme of tragedies related to product quality are preventable with the diligence of practices like validation

Cutter Laboratories § Producing polio vaccine in 1955 § Over 200, 000 people inoculated with vaccine prepared by Cutter Laboratories that inadvertently contained the live polio virus instead of an inactive virus § Inactivation poorly understood, especially with respect to scale and increasing concentrations of virus § 200 children developed permanent paralysis; 10 die
Large Volume Parenterals (LVP) § Solutions that are injected into the body are known as parenterals § ensuring that these solutions are sterile is a major concern for the organizations that produce them § Parenterals, and other heat stable medicines, are typically sterilized by a process called terminal sterilization § the final packaged product is sterilized using any of a number of methods, including moist heat

LVP for Burn Wounds § In 1971, parenteral solutions used in burn wards were incompletely sterilized and thus contaminated with live Pseudomonas spp. Bacteria § Patients with severe burns were administered these solutions, with a large number of patients developing infections § More than 50 resultant deaths § Poor understanding of production autoclave process allowing residual bacteria to survive
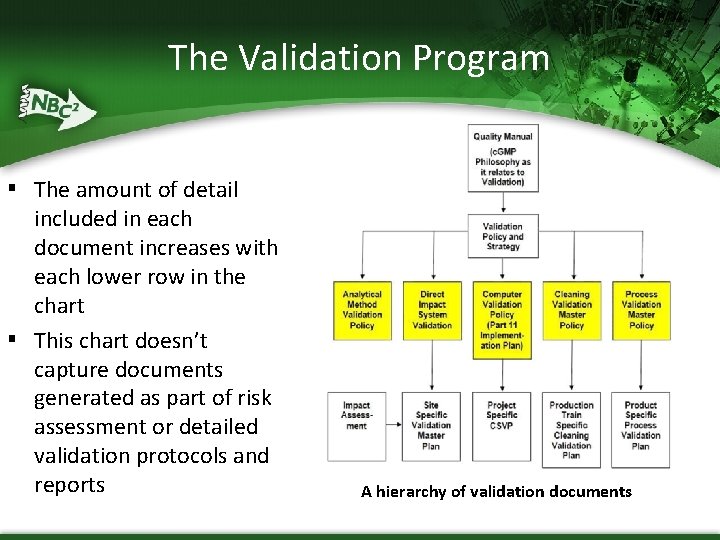
The Validation Program ▪ The amount of detail ▪ included in each document increases with each lower row in the chart This chart doesn’t capture documents generated as part of risk assessment or detailed validation protocols and reports A hierarchy of validation documents

Validation Master Policies § Increasing in specificity, Validation Master Policies are developed for those areas that require focused validation activities § These include validation of: § the analytical methods used to determine the purity or identity § § of raw materials, process intermediates, or final product systems that have a direct impact on product quality computer systems cleaning systems the overall manufacturing process

Validation Master Plans ▪ Validation Master Plans include specific information on individual areas and individual pieces of equipment within a facility ▪ The plans also include specifics on the type and extent of activities as they relate to each area or equipment type
Risk-based Validation § Risk analysis is a formal analytical activity § Identify § Assess § Manage § Risks considered in this process are related to § Product § Patient § Employees of the biomanufacturer

Identifying Systems § Develop a comprehensive list of all systems in the manufacturing operation, categorized by functional area § § facility, equipment, and utility systems analytical equipment systems computerized systems cleaning systems
Systems Impact Assessment (SIA) § The Systems Impact Assessment (SIA) is a process to determine which systems should be subject to qualification, which evaluates the impact that a system has on product quality § Each identified system is then categorized as one of the following § Direct Impact (DI) system § Indirect Impact (ID) system § No Impact (NI) system

Risk-based SIA ▪ Conducted by a multi-disciplinary team consisting of representatives from engineering, validation, operations, quality assurance, etc ▪ With the traditional approach toward validation, every system is qualified and validated ▪ With the risk-based approach, qualification activities are limited to Direct Impact Systems (DI)

Risk Assessment ▪ Patient safety: risk of a patient being physically harmed ▪ Product quality: risk that the product quality profile (identity, strength, quality, or purity) will be negatively impacted ▪ Compliance: risk of a regulatory enforcement action (e. G. , FDA, EMA, etc. ) Or the delay of a product approval
FMEA Method § § The initial framework is the failure of a process or process component Severity § Occurrence § Detection § Risk Priority Number § § Addresses the impact on a process in the event a parameter is out of range Or, it can address the impact on a patient if some product quality attribute is not met § Assesses the likelihood that the failure mode will occur § Addresses the ability of detecting the failure before it can cause the noted impact § RPN = Severity x Occurrence x Detection
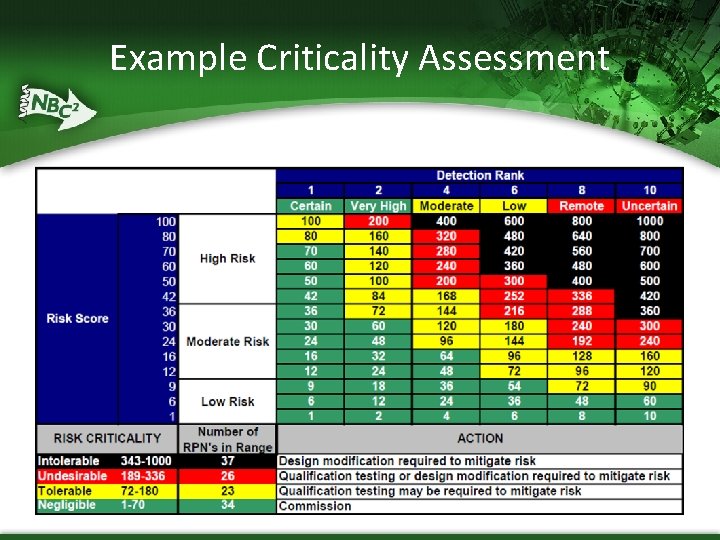
Example Criticality Assessment
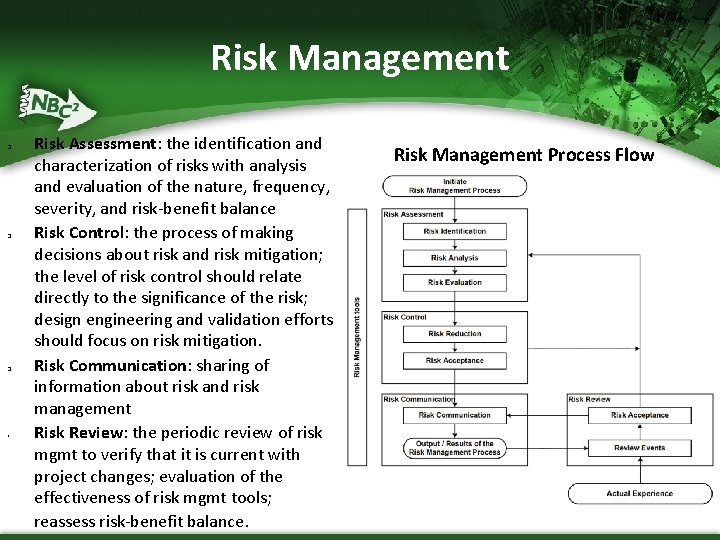
Risk Management q q q § Risk Assessment: the identification and characterization of risks with analysis and evaluation of the nature, frequency, severity, and risk-benefit balance Risk Control: the process of making decisions about risk and risk mitigation; the level of risk control should relate directly to the significance of the risk; design engineering and validation efforts should focus on risk mitigation. Risk Communication: sharing of information about risk and risk management Risk Review: the periodic review of risk mgmt to verify that it is current with project changes; evaluation of the effectiveness of risk mgmt tools; reassess risk-benefit balance. Risk Management Process Flow
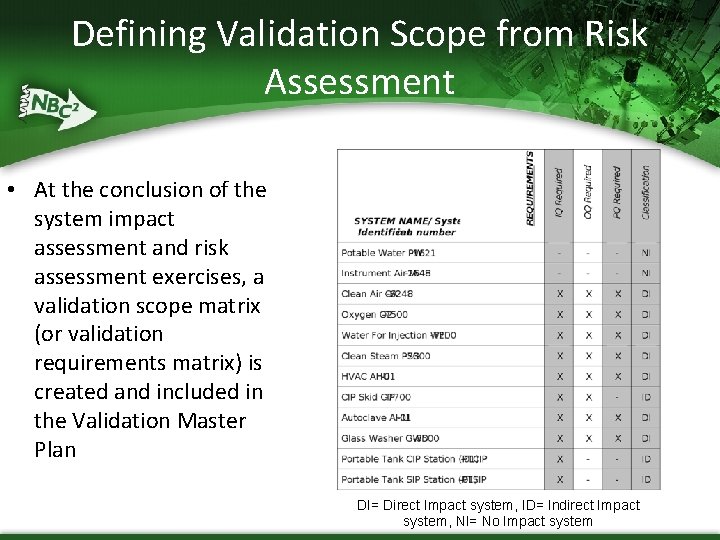
Defining Validation Scope from Risk Assessment • At the conclusion of the system impact assessment and risk assessment exercises, a validation scope matrix (or validation requirements matrix) is created and included in the Validation Master Plan DI= Direct Impact system, ID= Indirect Impact system, NI= No Impact system

Defining Individual Validation Plans § Develop individual validation plans for each system listed in the validation requirements matrix, corresponding to the level of qualification listed in the matrix § Purpose of this document is to define the various aspects of the equipment that need testing and to qualify each particular piece of equipment § Individual validation plan does not specify how the components are to be tested § Specified in the individual validation protocols
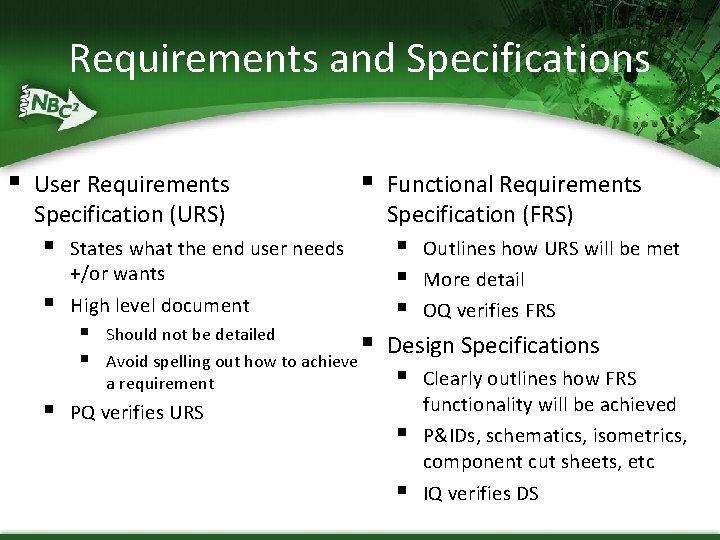
Requirements and Specifications § User Requirements Specification (URS) § § Should not be detailed Avoid spelling out how to achieve a requirement PQ verifies URS Functional Requirements Specification (FRS) § § § States what the end user needs +/or wants High level document § § § Outlines how URS will be met More detail OQ verifies FRS Design Specifications § § § Clearly outlines how FRS functionality will be achieved P&IDs, schematics, isometrics, component cut sheets, etc IQ verifies DS

Validation of Facilities, Equipment, and Utilities § Facility qualification and validation activities will establish and provide documented evidence that: § § § the premises, supporting utilities, equipment, and processes have been designed in accordance with c. GMP requirements; this constitutes Design Qualification (DQ). the premises, supporting utilities, and equipment have been built and installed in compliance with their design specifications; this constitutes Installation Qualification (IQ). the facilities, supporting utilities, and equipment operate in accordance with their design specifications; this constitutes Operational Qualification (OQ). the facilities, utilities, or equipment that can affect product quality perform as intended, meeting predetermined acceptance criteria; this constitutes Equipment Performance Qualification (PQ). a specific process will consistently produce a product meeting predetermined specifications and quality attributes; this constitutes Process Validation (PV); it can only be initiated once the facility has been validated (IQ + OQ + PQ).

Stages of Qualification/Validation ▪ Qualification stages begin with the design of the facility, equipment, or process ▪ Progresses through the ability of the facility, equipment, and process to produce a product meeting the predetermined product specifications ▪ The completion of the required qualification and/or validation stages for equipment/systems "cements" that equipment or operation in a validated state ▪ Any changes to the equipment or operation from that point forward may require re-validation
Requirements Phase § Must specify requirements for individual aspects of the facility, equipment, utility, and systems that address § § Function Throughput Operability Applicable compliance standards § This enables the development and assessment of specific engineering options § These requirements are usually formalized in a detailed User Requirement Specification (URS) document

Design Qualification (DQ) § The DQ confirms the functional design of the system as correct and appropriate given the requirements in the URS § A formal DQ protocol includes detailed comparisons of the functional design with § § regulatory requirements company procedures manufacturer’s documentation the URS

Extent of Validation and Qualification § A typical biopharmaceutical company can expect to qualify and validate the following for a new or upgraded manufacturing facility: § § critical process support utilities (e. g. , HVAC, compressed air, specialty gases, clean steam, and purified water systems) process equipment design, installation, and operation § DQ is the final step to formally review and document the proper system design before release to fabrication and construction

Construction § The materials developed to this point serve as a reference for the vendor and a contractual obligation to provide documentation such as, § § § material certifications for product contact surfaces welding information operation and maintenance manuals piping and instrumentation diagrams (P&IDs) shop drawings cut sheets/data sheets instrumentation calibration data sheets filter certifications schedules for construction and testing of equipment any related quality documents These are all included as part of a Turnover Package (TOP)
Commissioning § A well planned, documented, and managed engineering approach to § the start-up and turnover of facilities, systems, and equipment to the end-user or validation that results in a safe and functional environment that meets established design requirements and stakeholder expectations “Commissioning execution” typically involves § system inspection (visual testing) § adjustment and regulation § testing (individual system tests) § performance testing (combined system tests or sequence of operations testing)

Leveraging Commissioning Activities § When possible the commissioning activities should be well documented in order to “leverage” the test data forward as part of IQ/OQ testing § Can help save time, efforts, and cost later § For example, testing of the control loop on the dissolved oxygen control for a bioreactor is required as part of the risk assessment process and validation requirements matrix § This testing can be done as part of the commissioning process and, if appropriately documented and reviewed, used to satisfy OQ testing requirements

FAT and SAT § When equipment is ready to be delivered to the facility, the equipment manufacturer will schedule the Factory Acceptance Test (FAT) § § All safety and quality-critical items should be examined and documented and all of the defined documentation requirements should be assembled into a Turnover Package (TOP) Operational FATs can contribute to the OQ effort § Site Acceptance Testing (SAT) is additional testing done at the site of § use FAT and SAT, appropriately documented, can support and satisfy IQ and OQ testing requirements

Installation Qualification (IQ) § § Answers the questions: Do you know what you have? § Verified drawings § Manufacturer’s documentation § Critical components § Do you know what it needs in order to work? § Utilities § Compressed air, Clean Steam, electric § Environment § Room Classification, Temperature, RH § Workmanship § Level, properly supported, adequate clearance § Tied to Design Specs

Installation Qualification (IQ) and IQ Protocol § § IQ ensures that a piece of equipment is installed correctly Typical information in an IQ protocol § § § § Name and description of equipment, including model numbers and materials of construction Identification, including model and serial numbers Location of the equipment, drawings (P&ID) Any utility requirements, i. e. electrical voltage, steam or water pressure, etc. Any safety features of the equipment, including alarms, interlocks, or relief valves Controls (PLC, CPU, DCS) That all documentation, including manufacturers contact information, spare parts inventory, operational manual, and installation drawings are available on site

Installation Qualification • • • How is it achieved Drawing walk-downs Part cut-sheets Initial calibration Vendor certificates TOP Review • • What could go wrong Utilities piped wrong Instrumentation ranged or calibrated wrong Rouging, sealing problem or early failure Access to critical components

Operational Qualification (OQ) Answers the question: ▪ Does the system or equipment work as advertised? – Typical verifications: • Flow rates • Pressures • Temperatures • Valve sequencing • Alarms and interlocks – Tied to the manufacturer’s design and specifications (FRS)

Operational Qualification (OQ) and OQ Testing § § OQ demonstrates that the equipment or system operates as intended Typical testing that occurs during the OQ should include: § § § documenting the version of the software that is being tested any revisions to the software that occur during testing (e. g. , changes made as a result of failed tests) sequence of operation testing (testing all control functionality of the equipment/system) alarm testing power outage testing Standard Operating Procedure and logbook verification (verify that the operational SOPs are indicative of operation of the equipment and systemredline document(s) as required)

Operational Qualification • • • What is critical What could go wrong User interface navigation • Critical alarms undefined Security and modes • Controls/PID issues Alarms and interlocks Power up/down and failure Recipe sequence verification

Qualification Protocols IQ and OQ § Normally required to be written for “direct impact” systems § Individual documents describing § § § system under consideration documentation deliverables testing plans acceptance criteria forms for recording the test results § This is all to ensure that a system is installed and operates in accordance with predetermined specifications

Acceptance Criteria Installation Qualification § As part of an IQ protocol, acceptance criteria are clearly specified, which detail an acceptable outcome to the IQ activity § Bioreactor IQ (example) § A typical bioreactor can expect to have a post installation review of P&IDs § If the bioreactor is large and is hard piped in place, the installed system is in agreement with specifications, materials and installation details shown on the P&ID. Discrepancies have been marked.
IQ Bioreactor Example § IQ verifications (not all inclusive – see note below) § § § Verify “As Built” Nameplate info for critical components § § § Piping and Instrumentation Diagram (P&ID) Matches manufacturer’s specifications Support documentation § § Passivation documentation Operation and Maintenance (O&M) documentation § § § Weld logs Material certification Critical Utilities § § § Clean Steam Gases (O 2, Compressed Air, CO 2, N 2) Purified Water NOTE: These verifications must be tied to the User’s Requirements and the Manufacturer’s Design Specifications

OQ Bioreactor Example § OQ verifications (not all § Valve sequencing § Temperature control § Headspace pressure § Normal Operation § SIP § Cleaning inclusive – see IQ note) control § Sparged gas control § Flow and pressure § Agitator speed control § p. H control (cascade) § DO control (cascade)

Acceptance Criteria Example Operational Qualification Pressure Hold Test • Not more than 1 psi (0. 07 bar) is lost in ten minutes after pressurization to 10 psig (0. 7 barg) Temperature Control • Thermocouples show that the temperature can be raised to the set point, and maintained at that setpoint. • Thermocouples show that the temperature can be lowered to the set point, and maintained at that setpoint. Growth Parameter Control (p. H, O 2, CO 2) • Fermentor calibrated instrumentation shows that the various parameters can be raised to their setpoints, and maintained at those setpoints. • Fermentor calibrated instrumentation shows that the various parameters can be lowered to their setpoints, and maintained at those setpoints. Agitation Rate Empty Vessel Steam Sterilization • The agitator performs properly; the speed can be controlled. • Thermocouples are arranged throughout the vessel, including entry points, valves and difficult to penetrate locations. • Temperature uniformity within the chamber during steam sterilization, as shown by thermocouples, must be less than ± 1. 0°C of the mean chamber temperature. • Do not include the penetration thermocouples in valves and piping in the calculation. • Steam, during dwell in the empty chamber study, is saturated as shown by the temperature: pressure relationship.

Chromatography skid validation plan Installation Qualification (IQ) ▪ Separate IQ protocols are developed for each chromatography system ▪ The IQs will ensure that the system has been installed per design specifications and manufacturing requirements ▪ The IQ will include verification of all critical installation functions as identified in Documentation Requirements Scope Matrix

Chromatography skid validation plan Operational Qualification (OQ) § The OQ portion of the validation will consist of Documentation (software and design specs), Functional Testing, Sequence of Operation Testing, and Alarm Testing § Tests will include: § Sequence of Operations Testing § Verify that the chromatography system’s sequence of operations function as specified § Control System Verifications § Verify that the control system functions as specified § Testing will include security verifications, data entry/boundary limit verification, and operator interface display verifications § Data Integrity Testing § Verify that the chromatography skids satisfy requirements with respect to data integrity, trending, archival, and retrieval
Chromatography Skid OQ Tests § § § Alarm/Interlock Verification § Confirm that the alarms and interlocks function as specified Pump Performance Testing § Confirm that the pumps operate as specified over the required operating range or range of intended use UV Detector Linearity Test § Verify that the chromatography skid UV detector is linear within the specified tolerances over the intended range of use Step Gradient Test § Confirm that the chromatography skid control’s stepped gradients are within the specified tolerances over the intended range of use Linear Gradient Test § Confirm that the chromatography skid control’s linear gradients are within the specified tolerances over the intended range of use Cleaning Procedure Verification § Verify that cleaning procedures for the chromatography skid are in place and run to completion (Cleaning Validation will be handled separately in this example)

Performance Qualification (PQ) § § Answers the question: Does the system or equipment consistently meet your needs? § § § Requires a clear statement of user requirements Verifies properly installed and operating equipment being operated by trained operators following approved procedures § IQ and OQ complete § Documented training § Approved SOPs The same equipment may have more that one PQ, depending on the needs of different processes § Tied to the end user’s URS

PQ is a Final/Integrated Qualification ▪ The PQ integrates procedures, personnel, systems, and materials to verify that the utility, environment, equipment, or support system produces the required output ▪ This output may be either a product contact utility (clean compressed air, purified water, etc. ) or an environmental system such as HVAC ▪ Only “direct impact” systems will be subject to PQ
Acceptance Criteria Example Performance Qualification Sterilization: Empty Vessel • Bacillus stearothermophilus biological indicators are such that the product of the 'D' value and the log of the population is at least 6 (e. g. , D = 1. 5, population = 104). 105 -106 is preferred for steam strips. • Biological indicators enumerate within specifications per USP 23 Monograph • Positive control biological indicators are positive after incubation at 50 -55°C for seven days. • The sterilization cycle provides a minimum cumulative F 0 of 12 minutes, at end of cycle dwell, at each temperature sensor • All biological indicators placed are inactive after incubation at 50 -55°C for seven days. Sterile Integrity § No contamination seen in media-filled fermentor vessel when run under active conditions for a period longer than the standard fermentation
PQ Bioreactor Example § PQ verifications § Sterilization § Empty § Media (sometimes) § All sterilization verifications must be performed 3 X using Biological Indicators § Sterile hold § Sterilize media in vessel, hold for some period longer than what you want to claim, take samples to show that sterility is maintained § Cleaning § A much more complicated subject than the single word would lead you to believe § How clean is clean enough? § Process specific verifications are usually performed as part of Process Validation

Example Sterile Filtration Performance Qualification The PQ must demonstrate: ▪ ▪ ▪ ▪ ▪ All equipment in contact with the process fluids is chemically compatible and does not contaminate the product System integrity is maintained Passage of product (where appropriate) is sufficient Retention of product (where appropriate) is sufficient Passage of contaminants (where appropriate) is sufficient Retention of contaminants (where appropriate) is sufficient Recovery of the product is sufficient Total process time is in conformance with the design Finished drug product fulfills the product specifications Cleaning solutions remove all residual drug product, cells, and contaminants between processing runs

Related Programs § Additional validation activities would also be included in the Validation Master Plan (VMP) § § Cleaning Validation Sterilization Validation Analytical Method and Equipment Validation Software/Computer Validation
Validation of Analytical Methods and Equipment ▪ Validating the equipment used in the manufacturing process is a critical aspect of biopharmaceutical production ▪ No less important is validation of the analytical laboratory methods and equipment used in the QC process ▪ Analytical method validations are performed to confirm that the method(s) to be used on a qualified analytical instrument meet the specifications for the intended use
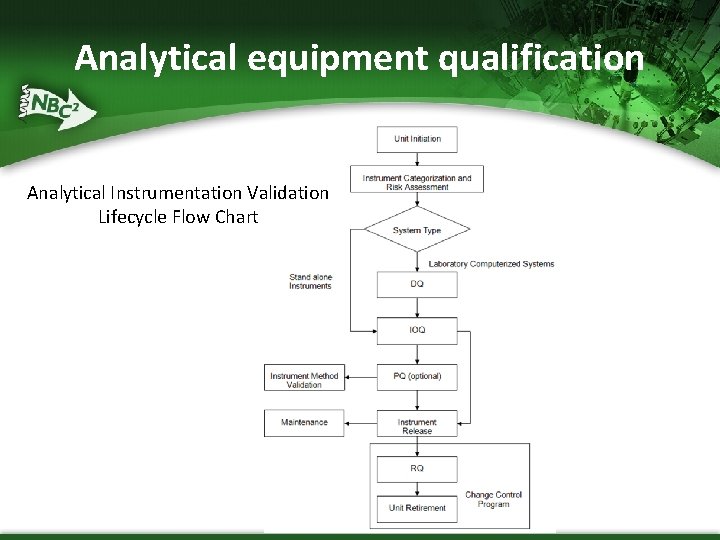
Analytical equipment qualification Analytical Instrumentation Validation Lifecycle Flow Chart

Analytical method qualification ▪ Qualification demonstrates that the method performs as expected before it is formally validated ▪ A qualified method should be capable of providing results consistent with proposed product specifications ▪ The following table indicates the performance characteristics that should be addressed during the qualification for the intended use of the method
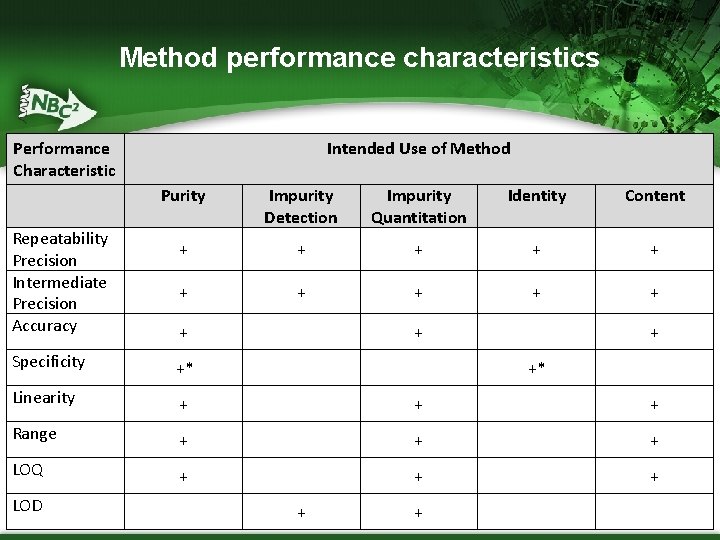
Method performance characteristics Performance Characteristic Repeatability Precision Intermediate Precision Accuracy Intended Use of Method Purity Impurity Detection Impurity Quantitation Identity Content + + + + Specificity +* Linearity + + + Range + + + LOQ + + + LOD +* + +
Aspects of the method ▪ For any analytical method there are several parameters that must be established for the method – selectivity or specificity of the method – demonstration of the linear range or validation of curve-fitting algorithms – Limit of Detection (LOD) of the method – Limit of Quantification (LOQ) of the method – the precision of the method (precision is covered in Chapter 3 Metrology)
Specificity § The specificity of a method refers to the method’s ability to detect the analyte in question in the presence of similar compounds § A method that has poor selectivity will give rise to frequent false positives, meaning the method falsely indicates that the substance being assessed is present
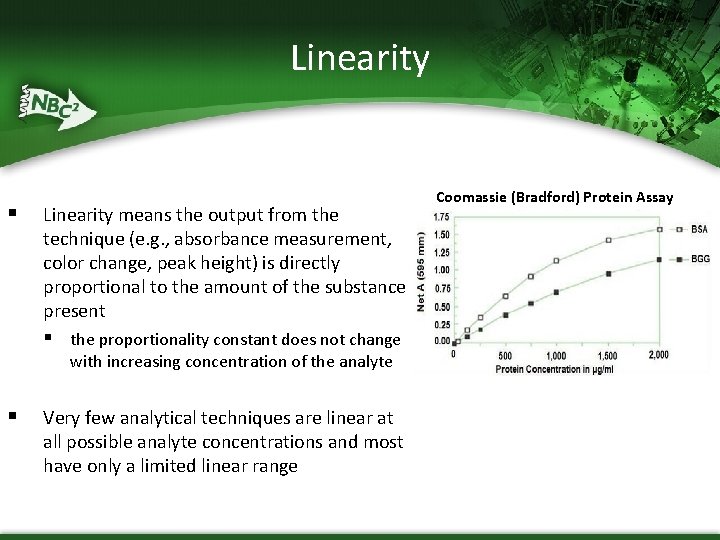
Linearity § Linearity means the output from the technique (e. g. , absorbance measurement, color change, peak height) is directly proportional to the amount of the substance present § the proportionality constant does not change with increasing concentration of the analyte § Very few analytical techniques are linear at all possible analyte concentrations and most have only a limited linear range Coomassie (Bradford) Protein Assay
Methods at their limits Limit of Detection (LOD) § Establishes the lower limit at which we can detect the analyte of interest § Useful in the measurement of impurities § We cannot often say that a preparation is zero levels of some analyte but only that it contains less than the LOD Limit of Quantification (LOQ) § Establishes the lower limit at which we can accurately quantify the analyte of interest § The presence of the analyte might be detected at a lower level, but because of various background fluctuations (including instrument and reagent variability) it is possible that an accurate quantification of the analyte cannot be made
Precision ▪ Precision refers to the variability of test results when the test is performed on multiple portions, or aliquots, of a homogenous sample ▪ To separate or distinguish factors responsible for methods variation, regulatory guidance suggests breaking precision into areas that include repeatability (or method precision) and system precision

Computerized Systems and Software Validation “the validation of systems to ensure accuracy, reliability, consistent intended performance, and the ability to discern invalid or altered records” § Validating computerized systems can result in a very complex and involved validation effort § CSV practices are not just internal to an organization— they extend to suppliers as well § Suppliers must assure quality and integrity in all aspects of their product
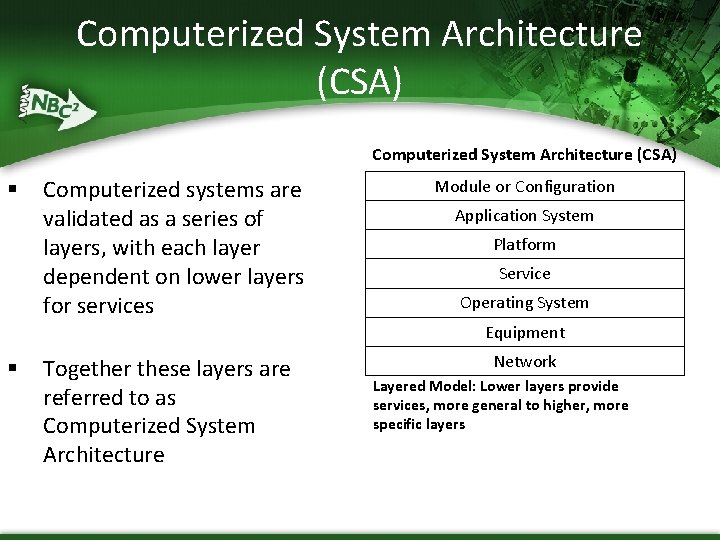
Computerized System Architecture (CSA) § Computerized systems are validated as a series of layers, with each layer dependent on lower layers for services Module or Configuration Application System Platform Service Operating System Equipment § Together these layers are referred to as Computerized System Architecture Network Layered Model: Lower layers provide services, more general to higher, more specific layers
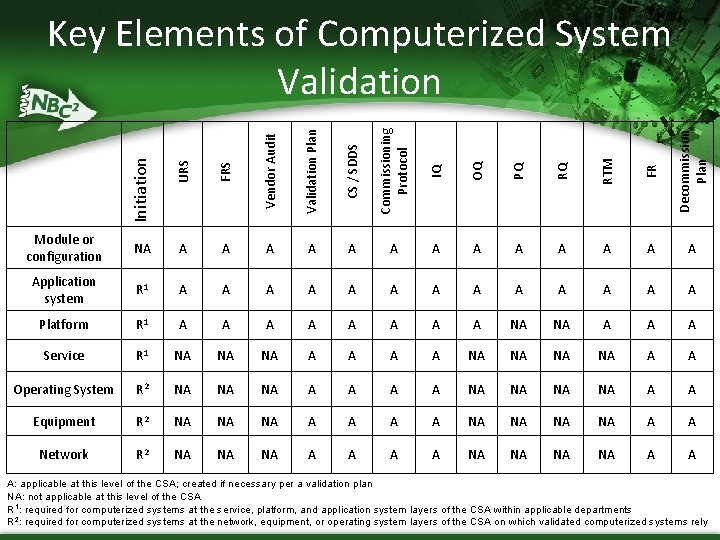
Initiation URS FRS Vendor Audit Validation Plan CS / SDDS Commissioning Protocol IQ OQ PQ RQ RTM FR Decommission Plan Key Elements of Computerized System Validation Module or configuration NA A A Application system R 1 A A A A Platform R 1 A A A A NA NA A Service R 1 NA NA NA A A Operating System R 2 NA NA NA A A Equipment R 2 NA NA NA A A Network R 2 NA NA NA A A A: applicable at this level of the CSA; created if necessary per a validation plan NA: not applicable at this level of the CSA R 1: required for computerized systems at the service, platform, and application system layers of the CSA within applicable departments R 2: required for computerized systems at the network, equipment, or operating system layers of the CSA on which validated computerized systems rely

Process Validation § Effective process validation contributes significantly to assuring drug quality § A drug should be produced that is fit for its intended use § To ensure this, the following conditions must exist § Quality, safety, and efficacy are designed or built into the product § Quality cannot be adequately assured merely by in-process and finished-product inspection or testing § Each step of a manufacturing process is controlled to assure that the finished product meets all quality attributes including specifications
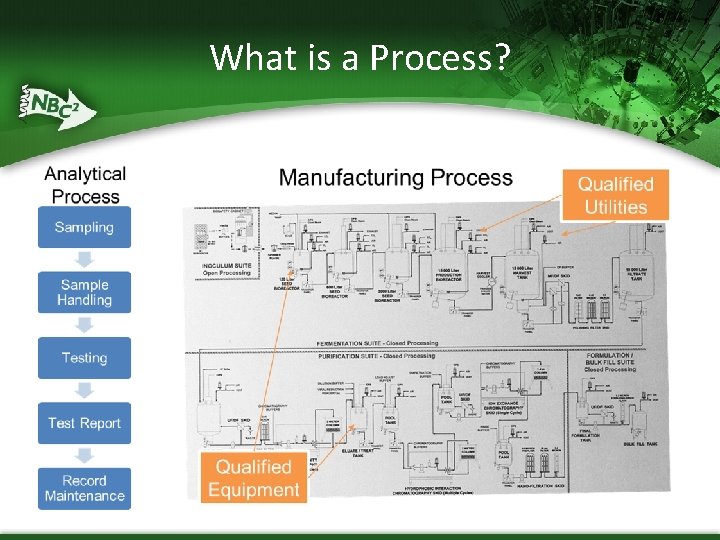
What is a Process?

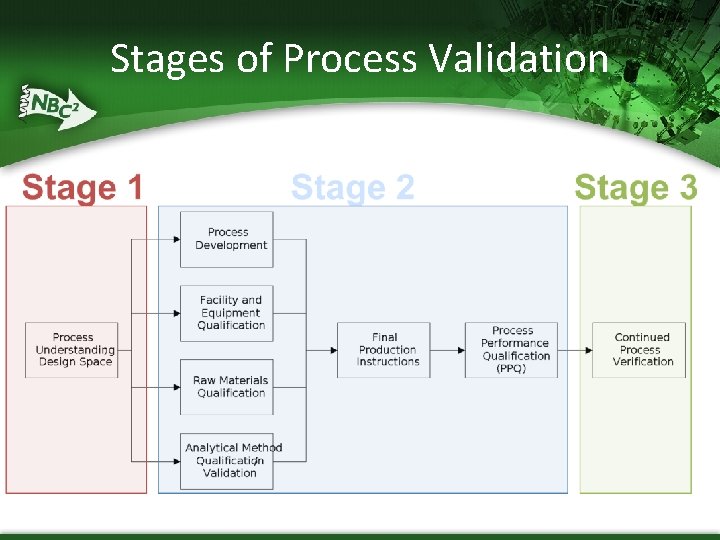
Approach to Process Validation (FDA Guidance) § Process Validation (PV) is defined as the collection and evaluation of data, from the process design stage through commercial production, which establishes scientific evidence that a process is capable of consistently delivering quality product § Stage 1 – Process Design § § Stage 2 – Process Qualification § § The commercial manufacturing process is defined during this stage based on knowledge gained through development and scale-up activities During this stage, the process design is evaluated to determine if the process is capable of reproducible commercial manufacturing Stage 3 – Continued Process Verification § Ongoing assurance is gained during routine production that the process remains in a state of control

Stage 1–Process Design § Building and Capturing Process Knowledge and Understanding § § § § typically performed at small-scale within non-GMP labs as part of developing (and locking) process determines effect of controllable parameters on various outputs defines key roles of individual process steps defines critical, key, and non-key parameters uses a Design of Experiments (DOE) approach in Upstream and Downstream processes provides data to further support Batch Record Ranges (Normal Operating Range; NOR) and Proven Acceptable Ranges (PAR) § This defines “Design Space” Establishing a Strategy for Process Control § Strategies for process control can be designed to (a) reduce input variation, (b) adjust for input variation during manufacturing (and so reduce its impact on the output), or (a+b)

Stage 2 – Process Qualification § Design of the facility and qualification of the equipment and utilities § Qualification activities (DQ, IQ, OQ, PQ) § Process performance qualification (PPQ) § The PPQ combines the actual facility, utilities, equipment (each now qualified), and the trained personnel with the commercial manufacturing process, control procedures, and components to produce commercial batches
Stage 3 – Continued Process Verification § Continual assurance that the process remains in a state of control (the validated state) during commercial manufacture § This is essentially captured through an existing well -functioning Quality System § Statistical process control (SPC) § CAPA § Change control
Stages of Process Validation
Key Upstream Processes to Validate § Cleaning § Bioreactors, media formulation tanks, centrifuge § Sterilization § Bioreactors, media, centrifuge § Master and Working Cell Banks § Fermentation/Cell Culture Process § Product Recovery
Upstream Validation Protocols Additional examples § Scale-down qualification § § verifying that the process operates the same at small-scale as it does at large-scale Media stability § verifying growth promotion for maximum hold duration § Stability of in-process intermediates at varying temperatures and maximum hold times § Cells at limit/end of production § § verifying that genetic consistency is maintained throughout maximum doublings at varying process parameters Conformance Lots: cell culture and harvest processes § demonstrating that the commercial process, when executed as specified in batch records, consistently produces in-process intermediates and Bulk Drug Substance (BDS) that meet all established specifications
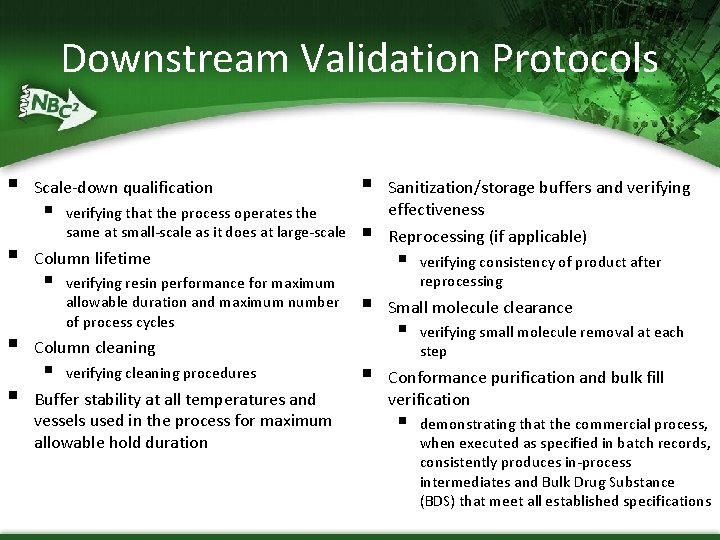
Downstream Validation Protocols § § Scale-down qualification § verifying that the process operates the same at small-scale as it does at large-scale Column lifetime § verifying resin performance for maximum allowable duration and maximum number of process cycles § § § Column cleaning § verifying cleaning procedures Buffer stability at all temperatures and vessels used in the process for maximum allowable hold duration § Sanitization/storage buffers and verifying effectiveness Reprocessing (if applicable) § verifying consistency of product after reprocessing Small molecule clearance § verifying small molecule removal at each step Conformance purification and bulk fill verification § demonstrating that the commercial process, when executed as specified in batch records, consistently produces in-process intermediates and Bulk Drug Substance (BDS) that meet all established specifications

Process Validation for Purifications § § § Small scale § § § Process characterization at small scale (determination of acceptable processing ranges) Sterile media and buffer stability (chemical, biochemical – but not microbiological) Initial resin and membrane reuse studies and hold studies (fresh and used) Process and product related impurities § § Clearance studies – virus, DNA, endotoxin § Product related: clipped or aggregated product, oxidation, disulfide pairing, posttranslational modification variants, any other product variants Removal studies (certain media components, leaching affinity ligand, leachables, extractables, any component that could be hazardous Full scale validation studies § § § Media and buffer storage in production vessels – microbiological Resin and membrane reuse at scale Resin and membrane lifetime
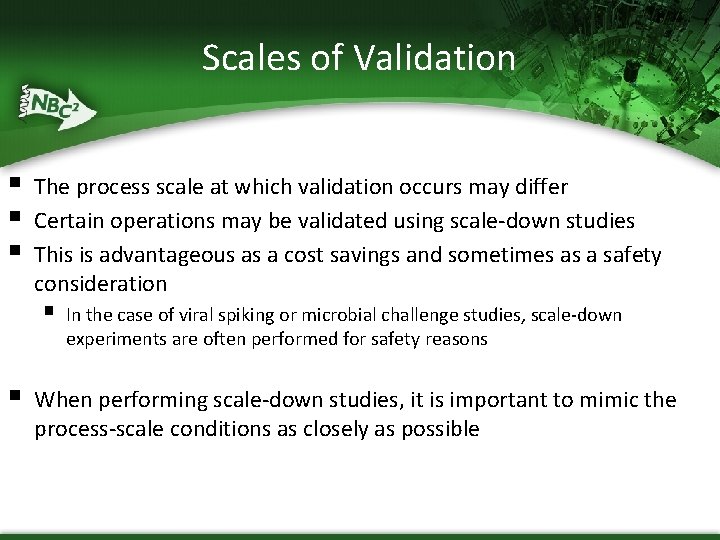
Scales of Validation § § § The process scale at which validation occurs may differ Certain operations may be validated using scale-down studies This is advantageous as a cost savings and sometimes as a safety consideration § § In the case of viral spiking or microbial challenge studies, scale-down experiments are often performed for safety reasons When performing scale-down studies, it is important to mimic the process-scale conditions as closely as possible
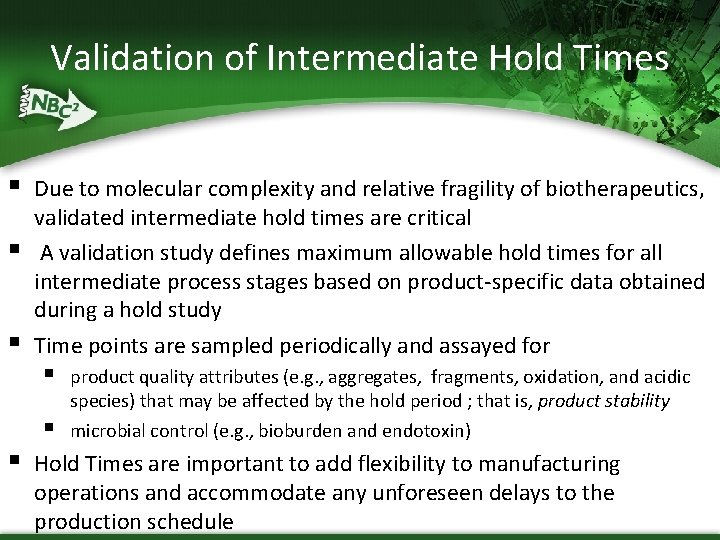
Validation of Intermediate Hold Times § § § Due to molecular complexity and relative fragility of biotherapeutics, validated intermediate hold times are critical A validation study defines maximum allowable hold times for all intermediate process stages based on product-specific data obtained during a hold study Time points are sampled periodically and assayed for § § § product quality attributes (e. g. , aggregates, fragments, oxidation, and acidic species) that may be affected by the hold period ; that is, product stability microbial control (e. g. , bioburden and endotoxin) Hold Times are important to add flexibility to manufacturing operations and accommodate any unforeseen delays to the production schedule

Three A Magic Number? § A historical credo stated that any one acceptable outcome is § possible, two acceptable outcomes are a coincidence, and three acceptable outcomes is a validated process Companies would strive to demonstrate that three batches of product through the manufacturing process demonstrated a validated process § Is this a high degree of assurance? § Increasingly, the process data determines validation § Is variability low? § Is the process well-controlled within acceptable limits?

Cleaning Validation ▪ The goal of the cleaning program is to deliver processes that are capable of consistently and effectively reducing potential by-product, and/or cleaning agent residues to below predetermined acceptance limits (criteria)
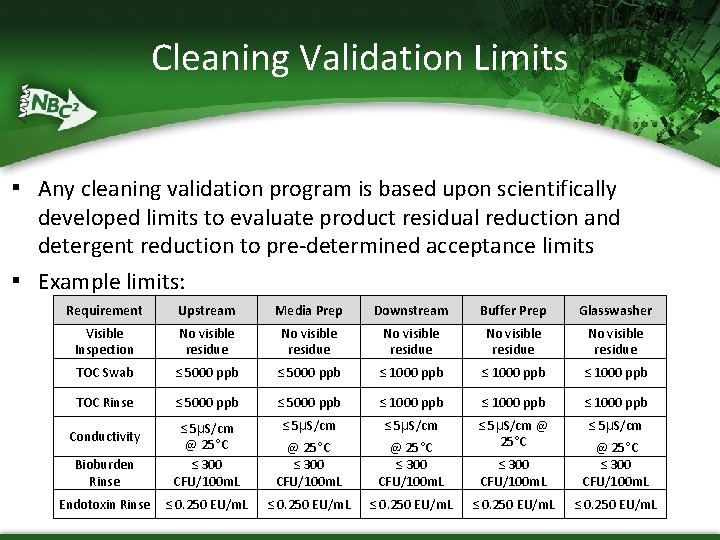
Cleaning Validation Limits ▪ Any cleaning validation program is based upon scientifically developed limits to evaluate product residual reduction and detergent reduction to pre-determined acceptance limits ▪ Example limits: Requirement Upstream Media Prep Downstream Buffer Prep Glasswasher Visible Inspection No visible residue No visible residue TOC Swab ≤ 5000 ppb ≤ 1000 ppb TOC Rinse ≤ 5000 ppb ≤ 1000 ppb ≤ 5μS/cm @ 25°C ≤ 300 CFU/100 m. L ≤ 5μS/cm @ 25°C Bioburden Rinse ≤ 5μS/cm @ 25°C ≤ 300 CFU/100 m. L Endotoxin Rinse ≤ 0. 250 EU/m. L Conductivity ≤ 300 CFU/100 m. L @ 25°C ≤ 300 CFU/100 m. L ≤ 0. 250 EU/m. L

Cleaning Processes § Most organizations validate three processes for cleaning equipment and systems § automated cleaning (Clean in Place or Clean out of Place) § semi-automated cleaning (SOP-driven, using mechanical methods) § manual cleaning § Each of these processes requires the same evaluation of effectiveness

Dirty Hold Time ▪ Validation of the Dirty Hold Time (DHT) provides a window of time to clean soiled equipment/systems using the validated cleaning processes ▪ DHTs provide operational flexibility to strategically stage cleanings, accommodating manufacturing schedules while assuring cleaning within defined limits

Clean Hold Time ▪ Validation of the Clean Hold Time (CHT) defines the maximum timeframe a cleaned system/equipment may be held clean and used before a re-cleaning is required ▪ The CHT affords operations the flexibility to use cleaned equipment for manufacturing over a defined period of time
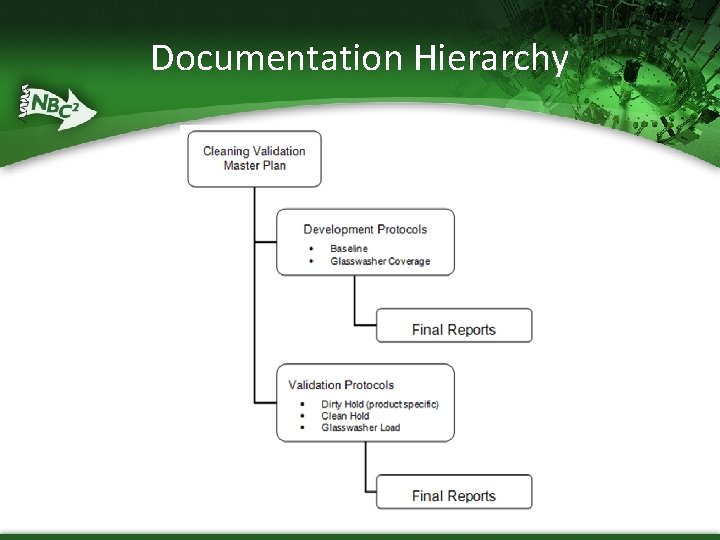
Documentation Hierarchy

Change Control and Re-validation § Changes—whether to procedures, documents, or equipment—must be controlled and evaluated for possible effects on product quality § Change control is a Quality requirement to assess impact of planned and unplanned process changes § Unplanned changes are typically a response to some failure § Planned changes are typically towards improvement § In all cases, the change is assessed in terms of impact on product quality and whether the change requires a revalidation

Out-of-Specification Results and Failure Investigations § At some point in the validation of a piece of equipment, method, process, or facility, a laboratory analysis will reveal an Out-of-Specification Result (OOS) § When this occurs, the written specification is not achieved § The failure investigation (root cause analysis) will involve various departments within the organization: production, facilities, QA, QC, etc. § SOPs and protocols will describe the collection of samples, such as where and how they are to be collected analyzed

Deviations § Reality says that nothing works perfectly § In validation, these problems are referred to as “deviations” § There are two kinds of deviations: § Procedural - You did something different from what you said you’d do in the protocol § Performance - The test result was not consistent with the protocol’s Acceptance Criteria

Procedural Deviation § Example: The protocol stated that you would qualify media sterilization in a bioreactor § After protocol approval, it was decided to sterile filter the media instead, so steam sterilization was not required § Resolution: Document this decision in a memo to the protocol file (the deviation), stating why it’s acceptable § Refer to this deviation in the relevant sections of the protocol
Performance Deviation § Example: Agitator speed range was stated as 5 to 100 rpm, with control ± 1 rpm § At execution, you found that the speed varied from 98 rpm to 103 rpm at the 100 rpm setpoint § Options: § You or the bioreactor manufacturer repairs, tunes, or replaces the agitator speed controls § You re-execute the test and document (in the deviation) that the speed control is now ± 1 rpm. § You review the process requirements and determine that ± 3 rpm is acceptable at the higher speed ranges § You document this review and state why it’s acceptable in the deviation

Deviations § Remember – these rationales may be reviewed by extremely skeptical people (Auditors) five years after the tests are done. § Deviations are not necessarily bad – they reflect reality § They must be resolved clearly and logically, however § “Perfect” protocols are suspect

Group Activity How does a Validation Program address these? §Safe and Effective Product >
- Slides: 99