Chapter 4 Types of Chemical Reactions and Solution




































- Slides: 76

Chapter 4 Types of Chemical Reactions and Solution Stoichiometry

Chapter 4 Table of Contents 4. 1 4. 2 4. 3 4. 4 4. 5 4. 6 4. 7 4. 8 4. 9 4. 10 Water, the Common Solvent The Nature of Aqueous Solutions: Strong and Weak Electrolytes The Composition of Solutions Types of Chemical Reactions Precipitation Reactions Describing Reactions in Solution Stoichiometry of Precipitation Reactions Acid–Base Reactions Oxidation–Reduction Reactions Balancing Oxidation–Reduction Equations Copyright © Cengage Learning. All rights reserved 2

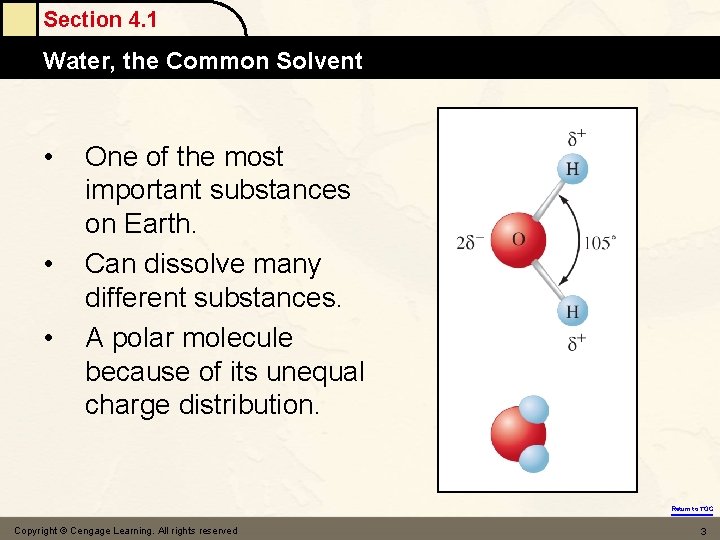
Section 4. 1 Water, the Common Solvent • • • One of the most important substances on Earth. Can dissolve many different substances. A polar molecule because of its unequal charge distribution. Return to TOC Copyright © Cengage Learning. All rights reserved 3

Section 4. 1 Water, the Common Solvent Dissolution of a Solid in a Liquid Return to TOC Copyright © Cengage Learning. All rights reserved 4

Section 4. 1 Water, the Common Solvent • The solubility of ionic substances in water varies greatly Na. Cl is quite soluble in water Ag. Cl is only very slightly soluble The differences in the solubilities of ionic compounds in water depend on the relative attractions of • The ions for each other • The ions for water molecules Return to TOC Copyright © Cengage Learning. All rights reserved 5

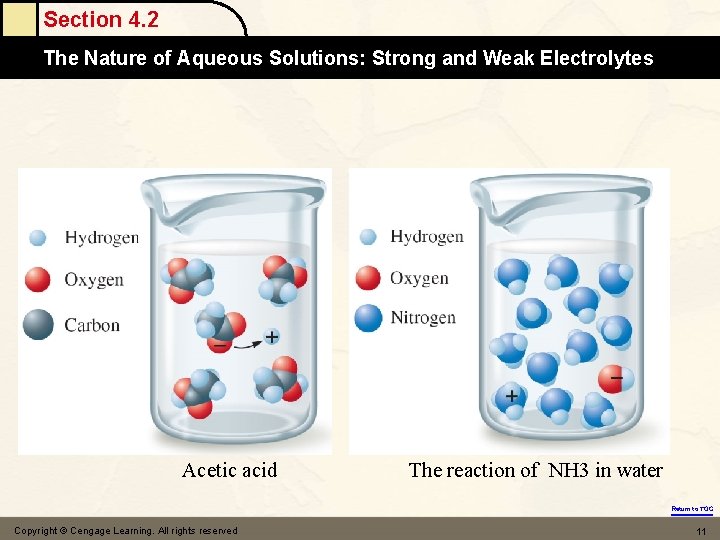
Section 4. 1 Water, the Common Solvent Water also dissolves many nonionic substances Ethanol(C 2 H 5 OH) 決定於樣本或兩相物質之化學本質,是屬極性或非 極性,而遵循『Like dissolves like(同質互溶) 』 的原則;即極性分子易溶入極性的固定相或流動相, 非極性分子則易溶入非極性者 Pure water will not dissolve animal fat(nonionic & nonpolar) Return to TOC Copyright © Cengage Learning. All rights reserved 6

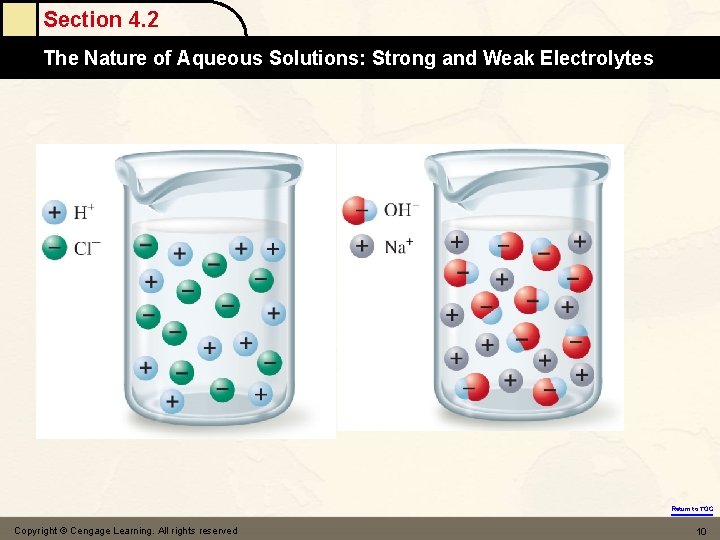
Section 4. 2 The Nature of Aqueous Solutions: Strong and Weak Electrolytes Nature of Aqueous Solutions • • • Solute – substance being dissolved. Solvent – liquid water. Electrolyte – substance that when dissolved in water produces a solution that can conduct electricity. Return to TOC Copyright © Cengage Learning. All rights reserved 7

Section 4. 2 The Nature of Aqueous Solutions: Strong and Weak Electrolytes • • • Strong Electrolytes – conduct current very efficiently (bulb shines brightly). Weak Electrolytes – conduct only a small current (bulb glows dimly). Nonelectrolytes – no current flows (bulb remains unlit). Return to TOC Copyright © Cengage Learning. All rights reserved 8

Section 4. 2 The Nature of Aqueous Solutions: Strong and Weak Electrolytes Na. Cl Acetic acid Sugar Return to TOC Copyright © Cengage Learning. All rights reserved

Section 4. 2 The Nature of Aqueous Solutions: Strong and Weak Electrolytes Return to TOC Copyright © Cengage Learning. All rights reserved 10

Section 4. 2 The Nature of Aqueous Solutions: Strong and Weak Electrolytes Acetic acid The reaction of NH 3 in water Return to TOC Copyright © Cengage Learning. All rights reserved 11

Section 4. 2 The Nature of Aqueous Solutions: Strong and Weak Electrolytes Electrolyte Behavior Return to TOC Copyright © Cengage Learning. All rights reserved 12

Section 4. 3 The Composition of Solutions Chemical Reactions of Solutions • We must know: § The nature of the reaction. § The amounts of chemicals present in the solutions. Return to TOC Copyright © Cengage Learning. All rights reserved 13

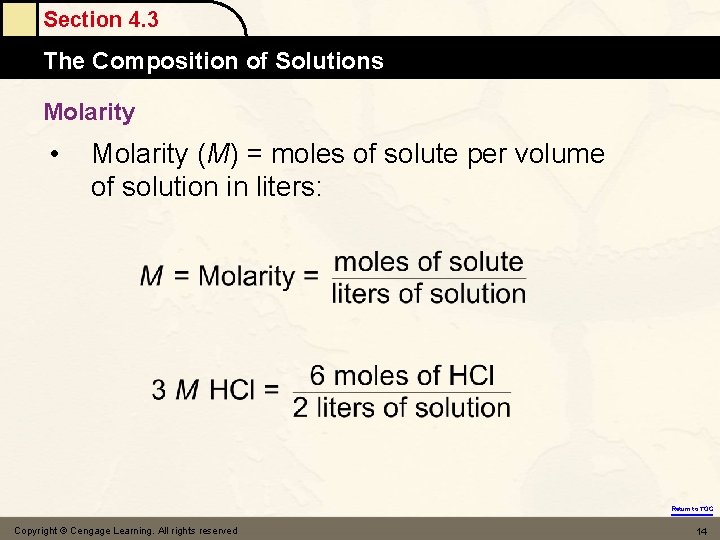
Section 4. 3 The Composition of Solutions Molarity • Molarity (M) = moles of solute per volume of solution in liters: Return to TOC Copyright © Cengage Learning. All rights reserved 14

Section 4. 3 The Composition of Solutions Exercise A 500. 0 -g sample of potassium phosphate is dissolved in enough water to make 1. 50 L of solution. What is the molarity of the solution? 500. 0 g / 212. 27 g/mol=2. 355 mole/1. 5 L=1. 57 M Return to TOC Copyright © Cengage Learning. All rights reserved 15

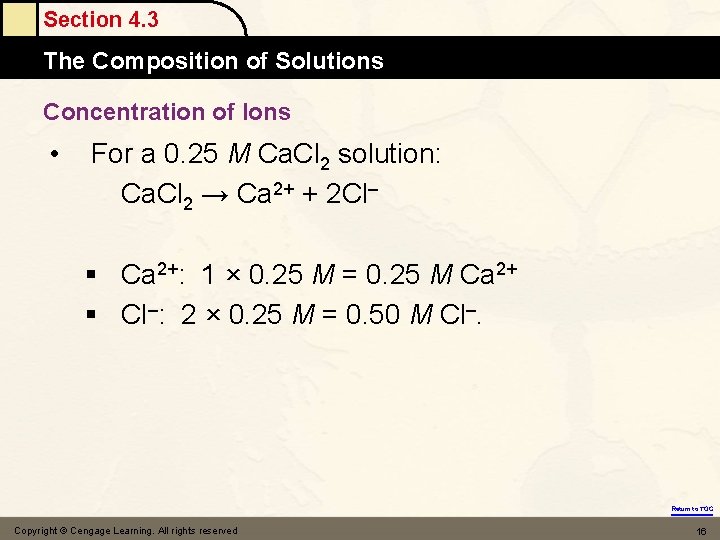
Section 4. 3 The Composition of Solutions Concentration of Ions • For a 0. 25 M Ca. Cl 2 solution: Ca. Cl 2 → Ca 2+ + 2 Cl– § Ca 2+: 1 × 0. 25 M = 0. 25 M Ca 2+ § Cl–: 2 × 0. 25 M = 0. 50 M Cl–. Return to TOC Copyright © Cengage Learning. All rights reserved 16

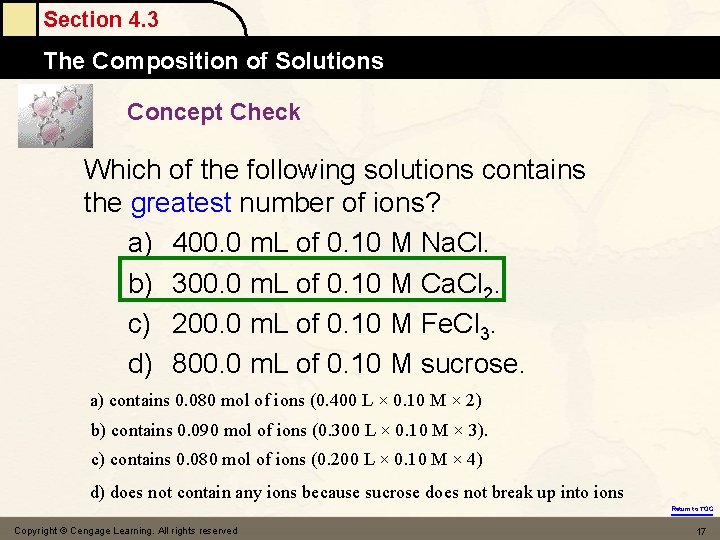
Section 4. 3 The Composition of Solutions Concept Check Which of the following solutions contains the greatest number of ions? a) 400. 0 m. L of 0. 10 M Na. Cl. b) 300. 0 m. L of 0. 10 M Ca. Cl 2. c) 200. 0 m. L of 0. 10 M Fe. Cl 3. d) 800. 0 m. L of 0. 10 M sucrose. a) contains 0. 080 mol of ions (0. 400 L × 0. 10 M × 2) b) contains 0. 090 mol of ions (0. 300 L × 0. 10 M × 3). c) contains 0. 080 mol of ions (0. 200 L × 0. 10 M × 4) d) does not contain any ions because sucrose does not break up into ions Return to TOC Copyright © Cengage Learning. All rights reserved 17

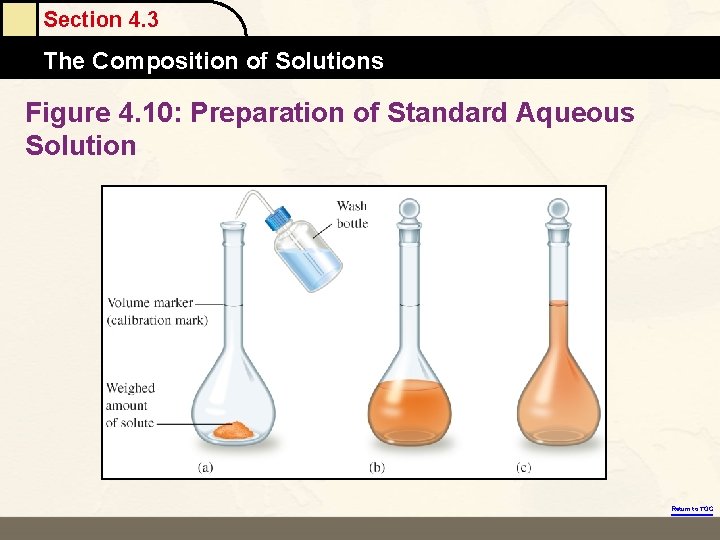
Section 4. 3 The Composition of Solutions Figure 4. 10: Preparation of Standard Aqueous Solution Return to TOC

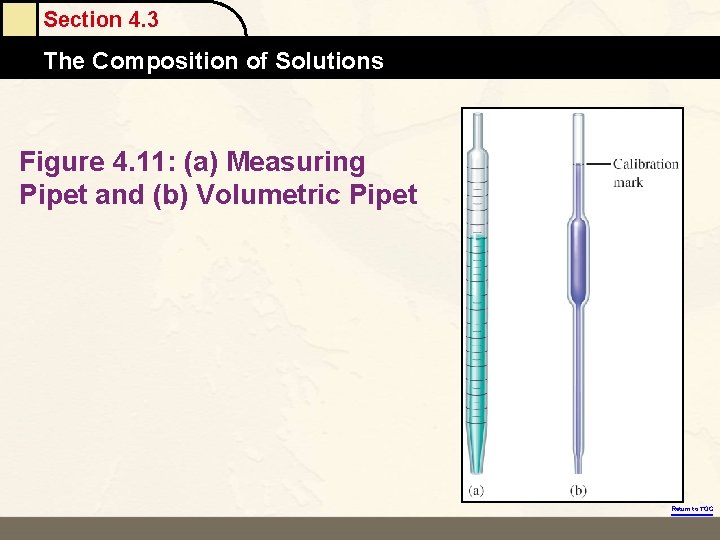
Section 4. 3 The Composition of Solutions Figure 4. 11: (a) Measuring Pipet and (b) Volumetric Pipet Return to TOC

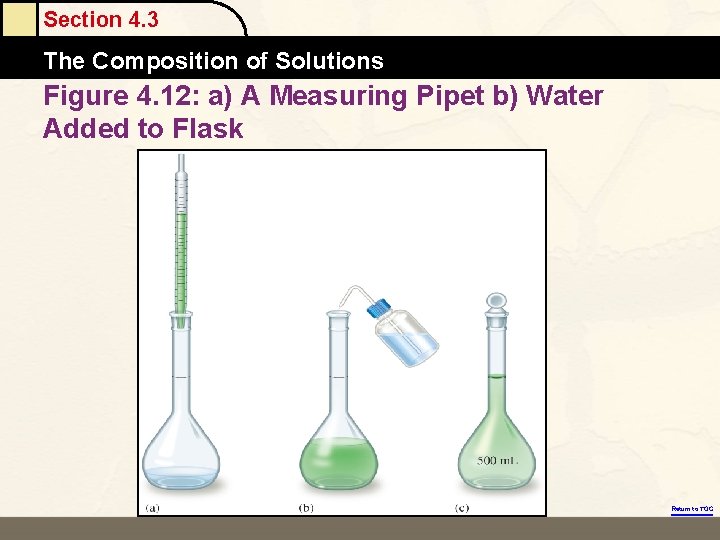
Section 4. 3 The Composition of Solutions Figure 4. 12: a) A Measuring Pipet b) Water Added to Flask Return to TOC

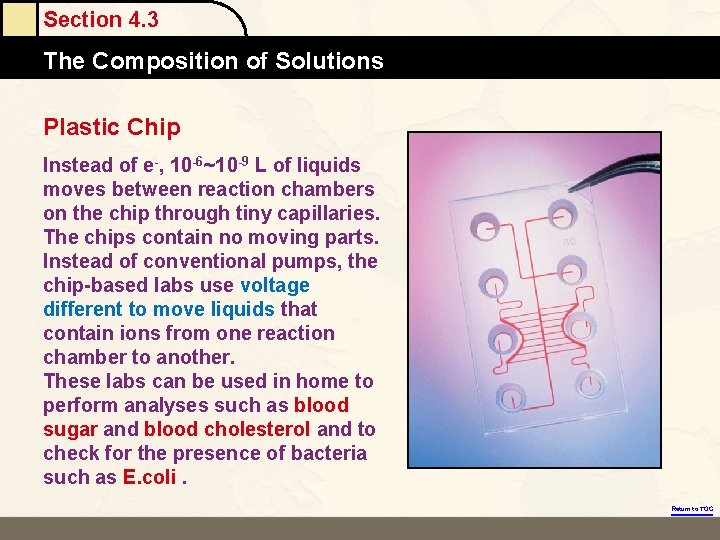
Section 4. 3 The Composition of Solutions Plastic Chip Instead of e-, 10 -6~10 -9 L of liquids moves between reaction chambers on the chip through tiny capillaries. The chips contain no moving parts. Instead of conventional pumps, the chip-based labs use voltage different to move liquids that contain ions from one reaction chamber to another. These labs can be used in home to perform analyses such as blood sugar and blood cholesterol and to check for the presence of bacteria such as E. coli. Return to TOC

Section 4. 3 The Composition of Solutions Dilution • • • The process of adding water to a concentrated or stock solution to achieve the molarity desired for a particular solution. Dilution with water does not alter the numbers of moles of solute present. Moles of solute before dilution = moles of solute after dilution M 1 V 1 = M 2 V 2 Return to TOC Copyright © Cengage Learning. All rights reserved 22

Section 4. 3 The Composition of Solutions Concept Check A 0. 50 M solution of sodium chloride in an open beaker sits on a lab bench. Which of the following would decrease the concentration of the salt solution? a) b) c) d) Add water to the solution. Pour some of the solution down the sink drain. Add more sodium chloride to the solution. Let the solution sit out in the open air for a couple of days. e) At least two of the above would decrease the concentration of the salt solution. Copyright © Cengage Learning. All rights reserved Return to TOC 23

Section 4. 3 The Composition of Solutions Exercise What is the minimum volume of a 2. 00 M Na. OH solution needed to make 150. 0 m. L of a 0. 800 M Na. OH solution? M V =M V 1 1 2 2 (2 M)(V ) = (0. 8 M)(150 m. L) 1 V 1= 60 m. L Return to TOC Copyright © Cengage Learning. All rights reserved 24

Section 4. 4 Types of Chemical Reactions • • • Precipitation Reactions Acid–Base Reactions Oxidation–Reduction Reactions Return to TOC Copyright © Cengage Learning. All rights reserved 25

Section 4. 5 Precipitation Reactions Precipitation Reaction • A double displacement reaction in which a solid forms and separates from the solution. § When ionic compounds dissolve in water, the resulting solution contains the separated ions. § Precipitate – the solid that forms. Return to TOC Copyright © Cengage Learning. All rights reserved 26

Section 4. 5 Precipitation Reactions Yellow Barium Chromate Richard Megna/Fundamental Photographs Return to TOC

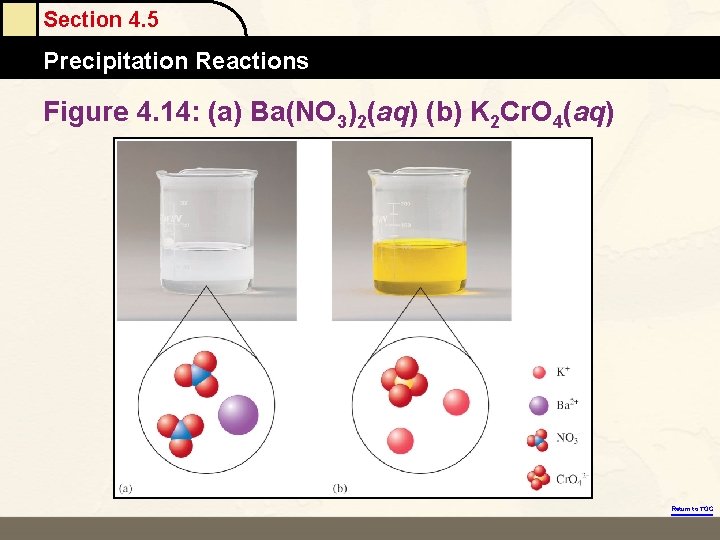
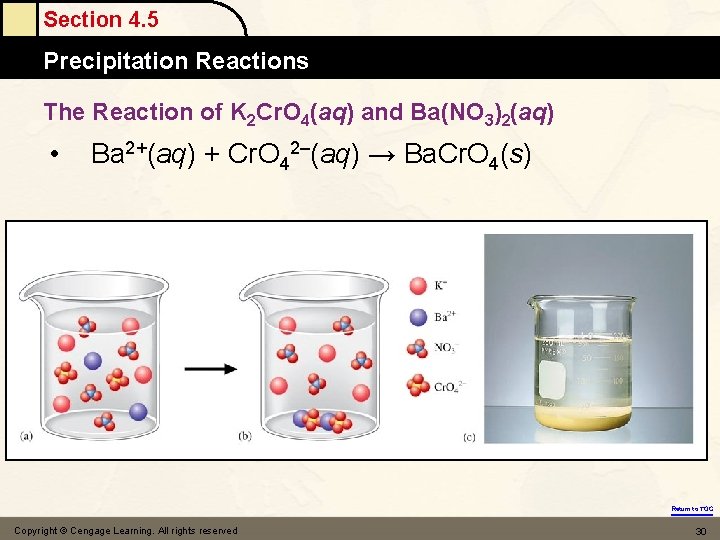
Section 4. 5 Precipitation Reactions Figure 4. 14: (a) Ba(NO 3)2(aq) (b) K 2 Cr. O 4(aq) Return to TOC

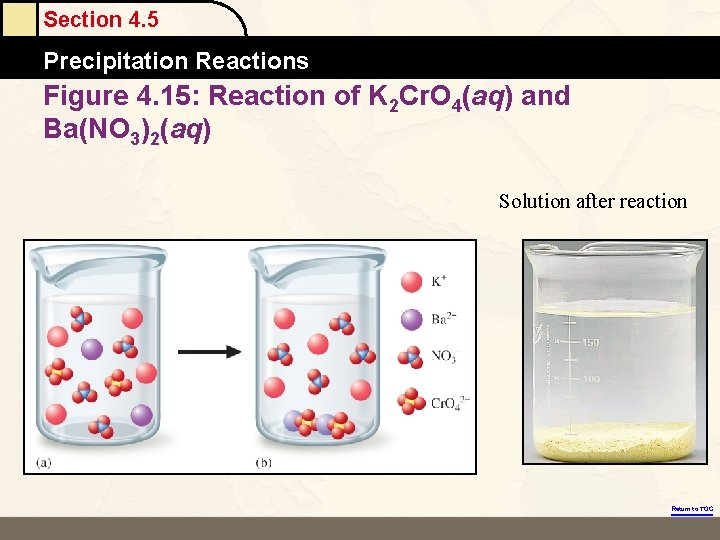
Section 4. 5 Precipitation Reactions Figure 4. 15: Reaction of K 2 Cr. O 4(aq) and Ba(NO 3)2(aq) Solution after reaction Return to TOC

Section 4. 5 Precipitation Reactions The Reaction of K 2 Cr. O 4(aq) and Ba(NO 3)2(aq) • Ba 2+(aq) + Cr. O 42–(aq) → Ba. Cr. O 4(s) Return to TOC Copyright © Cengage Learning. All rights reserved 30

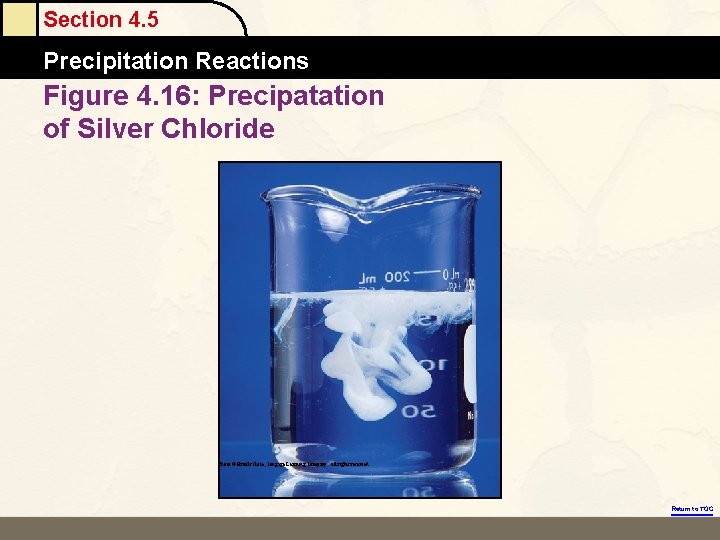
Section 4. 5 Precipitation Reactions Figure 4. 16: Precipatation of Silver Chloride Photo © Brooks/Cole, Cengage Learning Company. All rights reserved. Return to TOC

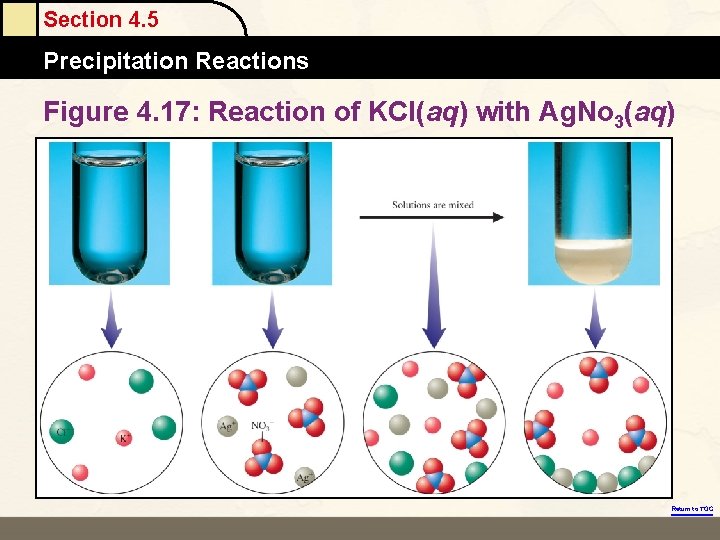
Section 4. 5 Precipitation Reactions Figure 4. 17: Reaction of KCl(aq) with Ag. No 3(aq) Return to TOC

Section 4. 5 Precipitation Reactions Precipitation of Silver Chloride Return to TOC Copyright © Cengage Learning. All rights reserved 33

Section 4. 5 Precipitation Reactions Precipitates • • • Soluble – solid dissolves in solution; (aq) is used in reaction. Insoluble – solid does not dissolve in solution; (s) is used in reaction. Insoluble and slightly soluble are often used interchangeably. Return to TOC Copyright © Cengage Learning. All rights reserved 34

Section 4. 5 Precipitation Reactions Simple Rules for Solubility 1. Most nitrate (NO 3 ) salts are soluble. 2. Most alkali metal (group 1 A) salts and NH 4+ are soluble. 3. Most Cl , Br , and I salts are soluble (except Ag+, Pb 2+, Hg 22+). 4. Most sulfate salts are soluble (except Ba. SO 4, Pb. SO 4, Hg 2 SO 4, Ca. SO 4). 5. Most OH are only slightly soluble (Na. OH, KOH are soluble, Ba(OH)2, Ca(OH)2 are marginally soluble). 6. Most S 2 , CO 32 , Cr. O 42 , PO 43 salts are only slightly soluble, except for those containing the cations in Rule 2. Copyright © Cengage Learning. All rights reserved Return to TOC 35

Section 4. 5 Precipitation Reactions Formation of Solid Fe(OH)3 Table 4. 1 indicate that both K+ and NO 3 - salts are soluble. However, Fe(OH)3 is only slightly soluble (Rule 5) Return to TOC

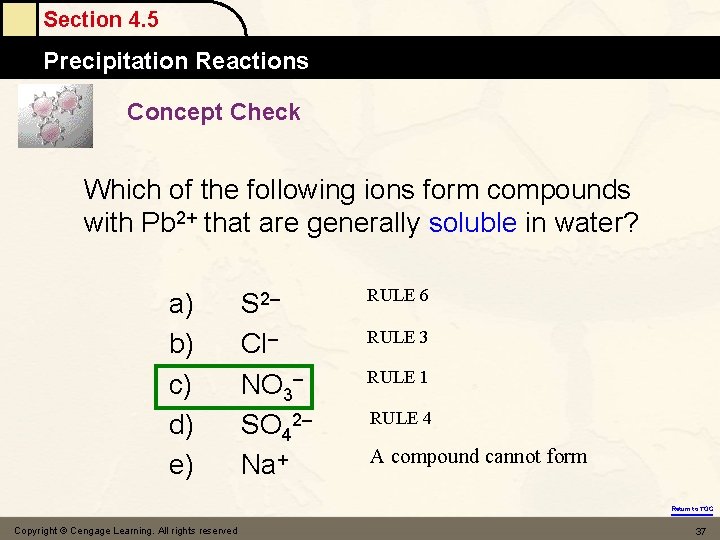
Section 4. 5 Precipitation Reactions Concept Check Which of the following ions form compounds with Pb 2+ that are generally soluble in water? a) b) c) d) e) S 2– Cl– NO 3– SO 42– Na+ RULE 6 RULE 3 RULE 1 RULE 4 A compound cannot form Return to TOC Copyright © Cengage Learning. All rights reserved 37

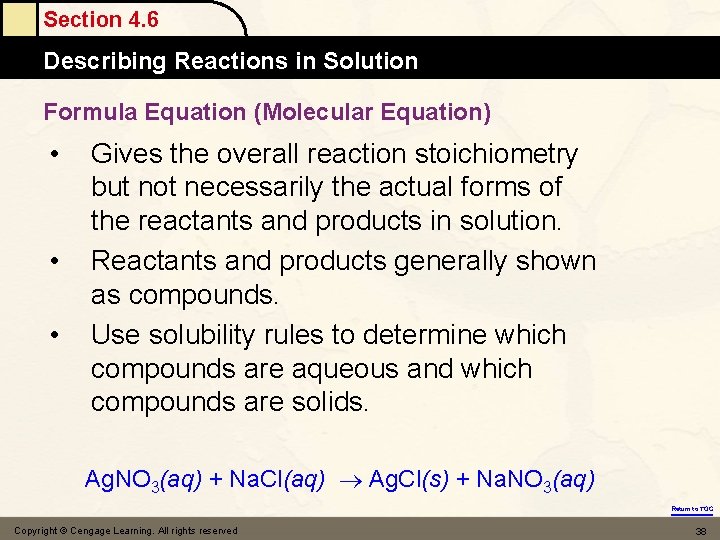
Section 4. 6 Describing Reactions in Solution Formula Equation (Molecular Equation) • • • Gives the overall reaction stoichiometry but not necessarily the actual forms of the reactants and products in solution. Reactants and products generally shown as compounds. Use solubility rules to determine which compounds are aqueous and which compounds are solids. Ag. NO 3(aq) + Na. Cl(aq) Ag. Cl(s) + Na. NO 3(aq) Return to TOC Copyright © Cengage Learning. All rights reserved 38

Section 4. 6 Describing Reactions in Solution Complete Ionic Equation • Represents as ions all reactants and products that are strong electrolytes. Ag+(aq) + NO 3 (aq) + Na+(aq) + Cl (aq) Ag. Cl(s) + Na+(aq) + NO 3 (aq) Return to TOC Copyright © Cengage Learning. All rights reserved 39

Section 4. 6 Describing Reactions in Solution Net Ionic Equation • Includes only those solution components undergoing a change. § Show only components that actually react. Ag+(aq) + Cl (aq) Ag. Cl(s) • Spectator ions are not included (ions that do not participate directly in the reaction). § Na+ and NO 3 are spectator ions. 完整的離子方程式顯示只有部分離子參與反應。溶液裡的鈉離子Na+和硝酸根離子 NO 3 -,不論反應前或反應後都一樣;這些離子並沒有直接參與反應,稱為「旁觀離 子 spectator ions」。 Return to TOC Copyright © Cengage Learning. All rights reserved 40

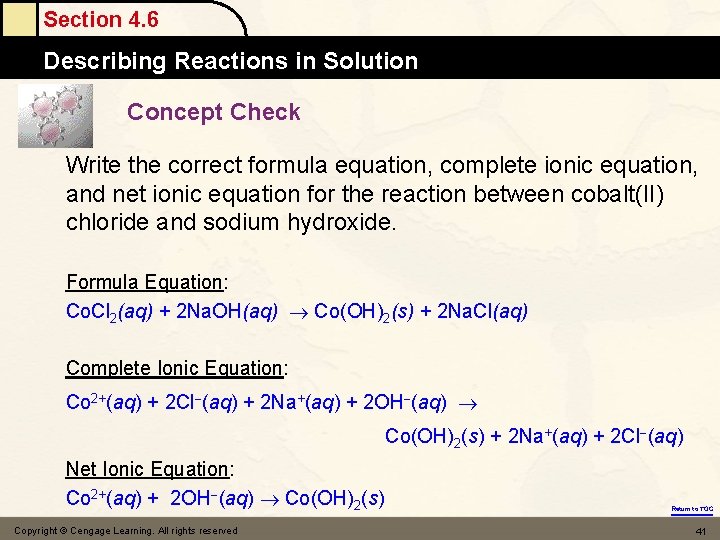
Section 4. 6 Describing Reactions in Solution Concept Check Write the correct formula equation, complete ionic equation, and net ionic equation for the reaction between cobalt(II) chloride and sodium hydroxide. Formula Equation: Co. Cl 2(aq) + 2 Na. OH(aq) Co(OH)2(s) + 2 Na. Cl(aq) Complete Ionic Equation: Co 2+(aq) + 2 Cl (aq) + 2 Na+(aq) + 2 OH (aq) Co(OH)2(s) + 2 Na+(aq) + 2 Cl (aq) Net Ionic Equation: Co 2+(aq) + 2 OH (aq) Co(OH)2(s) Copyright © Cengage Learning. All rights reserved Return to TOC 41

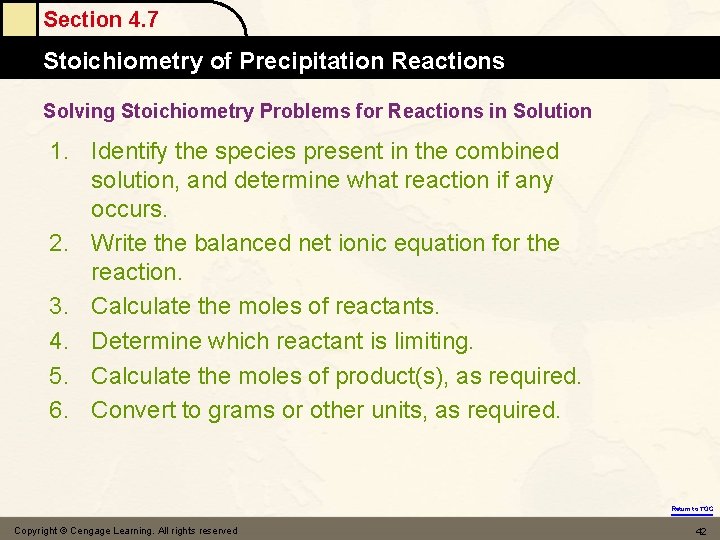
Section 4. 7 Stoichiometry of Precipitation Reactions Solving Stoichiometry Problems for Reactions in Solution 1. Identify the species present in the combined solution, and determine what reaction if any occurs. 2. Write the balanced net ionic equation for the reaction. 3. Calculate the moles of reactants. 4. Determine which reactant is limiting. 5. Calculate the moles of product(s), as required. 6. Convert to grams or other units, as required. Return to TOC Copyright © Cengage Learning. All rights reserved 42

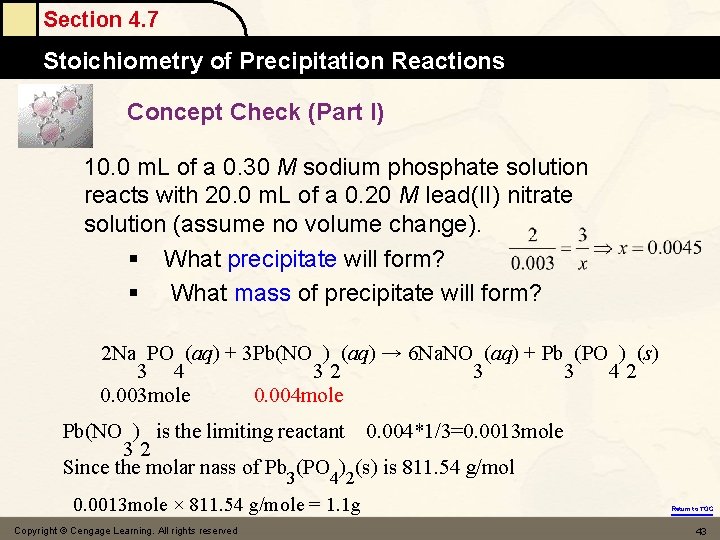
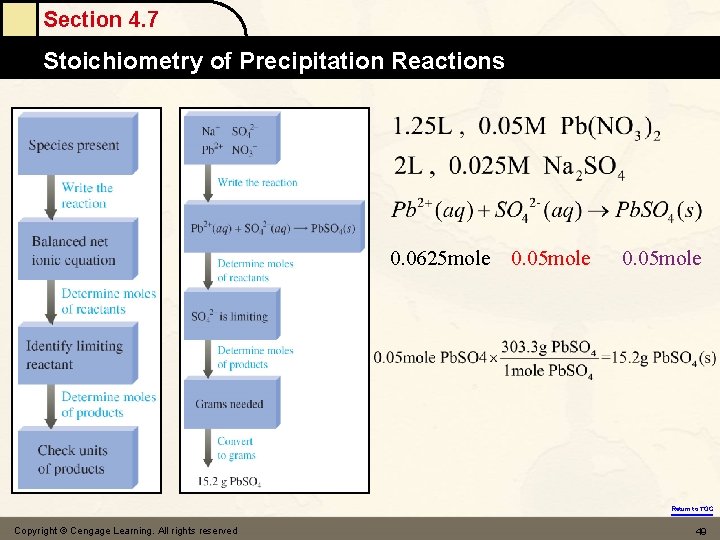
Section 4. 7 Stoichiometry of Precipitation Reactions Concept Check (Part I) 10. 0 m. L of a 0. 30 M sodium phosphate solution reacts with 20. 0 m. L of a 0. 20 M lead(II) nitrate solution (assume no volume change). § What precipitate will form? § What mass of precipitate will form? 2 Na PO (aq) + 3 Pb(NO ) (aq) → 6 Na. NO (aq) + Pb (PO ) (s) 3 4 32 3 3 42 0. 003 mole 0. 004 mole Pb(NO ) is the limiting reactant 0. 004*1/3=0. 0013 mole 32 Since the molar nass of Pb 3(PO 4)2(s) is 811. 54 g/mol 0. 0013 mole × 811. 54 g/mole = 1. 1 g Copyright © Cengage Learning. All rights reserved Return to TOC 43

Section 4. 7 Stoichiometry of Precipitation Reactions Let’s Think About It • Where are we going? § • To find the mass of solid Pb 3(PO 4)2 formed. How do we get there? § § § What are the ions present in the combined solution? What is the balanced net ionic equation for the reaction? What are the moles of reactants present in the solution? Which reactant is limiting? What moles of Pb 3(PO 4)2 will be formed? What mass of Pb 3(PO 4)2 will be formed? Return to TOC Copyright © Cengage Learning. All rights reserved 44

Section 4. 7 Stoichiometry of Precipitation Reactions Concept Check (Part II) 10. 0 m. L of a 0. 30 M sodium phosphate solution reacts with 20. 0 m. L of a 0. 20 M lead(II) nitrate solution (assume no volume change). § What is the concentration of nitrate ions left in solution after the reaction is complete? Nitrate ions are spectator ions and do not participate directly in the chemical reaction. Since there were 0. 004 mol of Pb(NO 3)2 present to start, then 0. 008 mol of nitrate ions are present. The total volume in solution is 30. 0 m. L. Therefore the concentration of nitrate ions = 0. 0080 mol / 0. 0300 L = 0. 27 M. Return to TOC Copyright © Cengage Learning. All rights reserved 45

Section 4. 7 Stoichiometry of Precipitation Reactions Let’s Think About It • Where are we going? § • To find the concentration of nitrate ions left in solution after the reaction is complete. How do we get there? § § What are the moles of nitrate ions present in the combined solution? What is the total volume of the combined solution? Return to TOC Copyright © Cengage Learning. All rights reserved 46

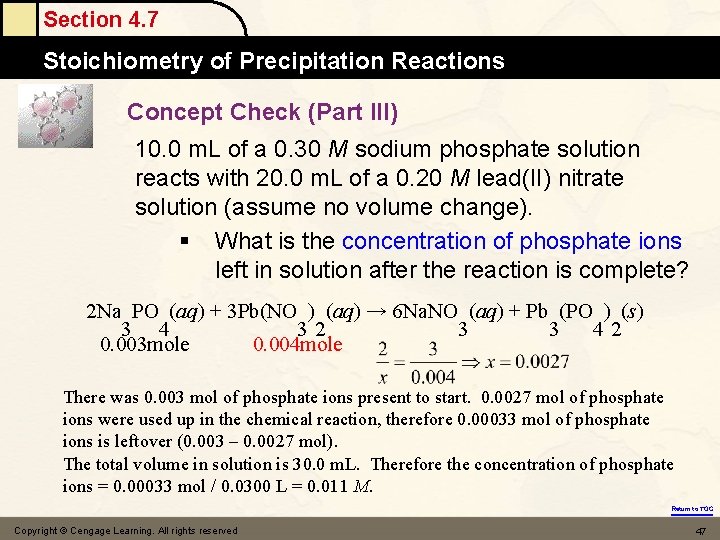
Section 4. 7 Stoichiometry of Precipitation Reactions Concept Check (Part III) 10. 0 m. L of a 0. 30 M sodium phosphate solution reacts with 20. 0 m. L of a 0. 20 M lead(II) nitrate solution (assume no volume change). § What is the concentration of phosphate ions left in solution after the reaction is complete? 2 Na PO (aq) + 3 Pb(NO ) (aq) → 6 Na. NO (aq) + Pb (PO ) (s) 3 4 32 3 3 42 0. 003 mole 0. 004 mole There was 0. 003 mol of phosphate ions present to start. 0. 0027 mol of phosphate ions were used up in the chemical reaction, therefore 0. 00033 mol of phosphate ions is leftover (0. 003 – 0. 0027 mol). The total volume in solution is 30. 0 m. L. Therefore the concentration of phosphate ions = 0. 00033 mol / 0. 0300 L = 0. 011 M. Return to TOC Copyright © Cengage Learning. All rights reserved 47

Section 4. 7 Stoichiometry of Precipitation Reactions Let’s Think About It • Where are we going? § • To find the concentration of phosphate ions left in solution after the reaction is complete. How do we get there? § § What are the moles of phosphate ions present in the solution at the start of the reaction? How many moles of phosphate ions were used up in the reaction to make the solid Pb 3(PO 4)2? How many moles of phosphate ions are left over after the reaction is complete? What is the total volume of the combined solution? Copyright © Cengage Learning. All rights reserved Return to TOC 48

Section 4. 7 Stoichiometry of Precipitation Reactions 0. 0625 mole 0. 05 mole Return to TOC Copyright © Cengage Learning. All rights reserved 49

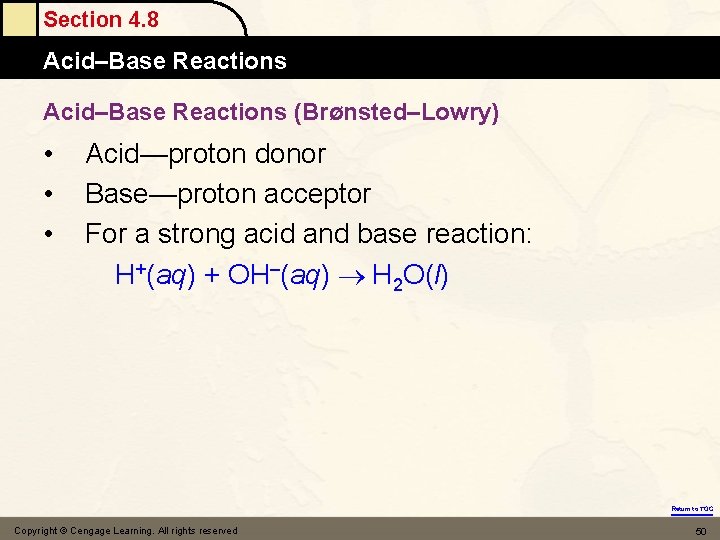
Section 4. 8 Acid–Base Reactions (Brønsted–Lowry) • • • Acid—proton donor Base—proton acceptor For a strong acid and base reaction: H+(aq) + OH–(aq) H 2 O(l) Return to TOC Copyright © Cengage Learning. All rights reserved 50

Section 4. 8 Acid–Base Reactions Neutralization of a Strong Acid by a Strong Base Return to TOC Copyright © Cengage Learning. All rights reserved 51

Section 4. 8 Acid–Base Reactions Performing Calculations for Acid–Base Reactions 1. List the species present in the combined solution before any reaction occurs, and decide what reaction will occur. 2. Write the balanced net ionic equation for this reaction. 3. Calculate moles of reactants. 4. Determine the limiting reactant, where appropriate. 5. Calculate the moles of the required reactant or product. 6. Convert to grams or volume (of solution), as required. Copyright © Cengage Learning. All rights reserved Return to TOC 52

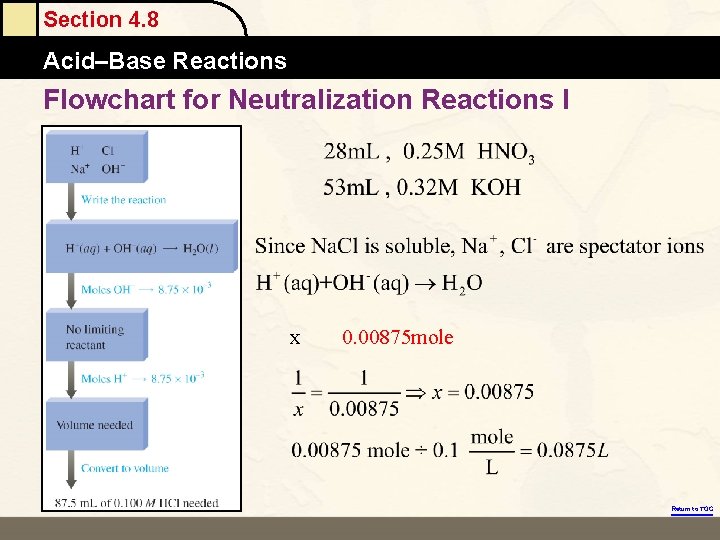
Section 4. 8 Acid–Base Reactions Flowchart for Neutralization Reactions I x 0. 00875 mole Return to TOC

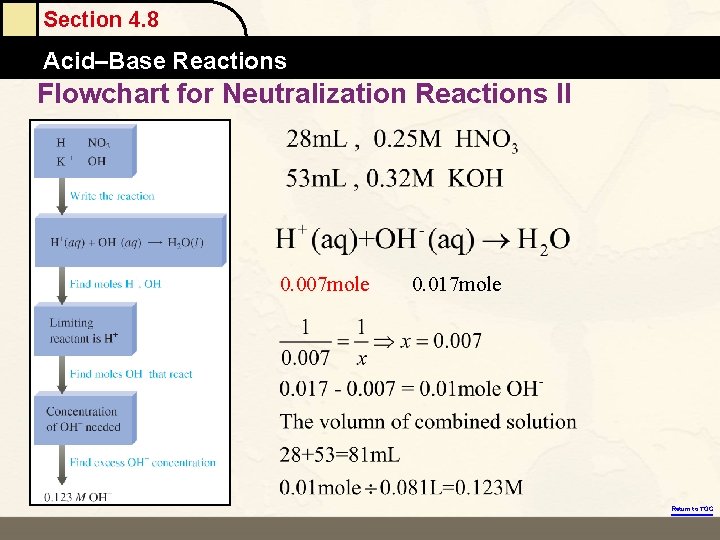
Section 4. 8 Acid–Base Reactions Flowchart for Neutralization Reactions II 0. 007 mole 0. 017 mole Return to TOC

Section 4. 8 Acid–Base Reactions Figure 4. 18: (a)(b)(c) Titration of Acid with a Base Return to TOC

Section 4. 8 Acid–Base Reactions Acid–Base Titrations • • • Titration – delivery of a measured volume of a solution of known concentration (the titrant) into a solution containing the substance being analyzed (the analyte). Equivalence point – enough titrant added to react exactly with the analyte. Endpoint – the indicator changes color so you can tell the equivalence point has been reached. Return to TOC Copyright © Cengage Learning. All rights reserved 56

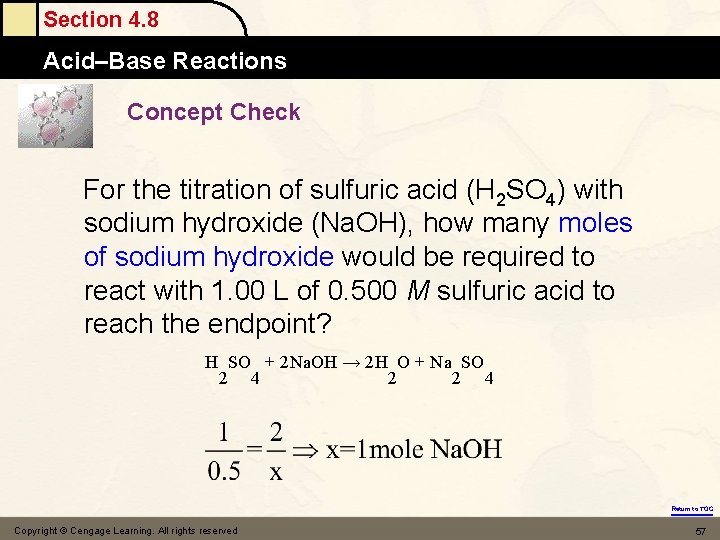
Section 4. 8 Acid–Base Reactions Concept Check For the titration of sulfuric acid (H 2 SO 4) with sodium hydroxide (Na. OH), how many moles of sodium hydroxide would be required to react with 1. 00 L of 0. 500 M sulfuric acid to reach the endpoint? H SO + 2 Na. OH → 2 H O + Na SO 2 4 2 2 4 Return to TOC Copyright © Cengage Learning. All rights reserved 57

Section 4. 8 Acid–Base Reactions Let’s Think About It • Where are we going? § • To find the moles of Na. OH required for the reaction. How do we get there? § § What are the ions present in the combined solution? What is the reaction? What is the balanced net ionic equation for the reaction? What are the moles of H+ present in the solution? How much OH– is required to react with all of the H+ present? Return to TOC Copyright © Cengage Learning. All rights reserved 58

Section 4. 9 Oxidation–Reduction Reactions Redox reactions are a family of reactions that are concerned with the transfer of electrons between species. Like acidbase reactions, redox reactions are a matched set -- you don't have an oxidation reaction without a reduction reaction happening at the same time. Oxidation refers to the loss of electrons, while reduction refers to the gain of electrons. Each reaction by itself is called a "half-reaction", simply because we need two (2) half-reactions to form a whole reaction. Return to TOC Copyright © Cengage Learning. All rights reserved 59

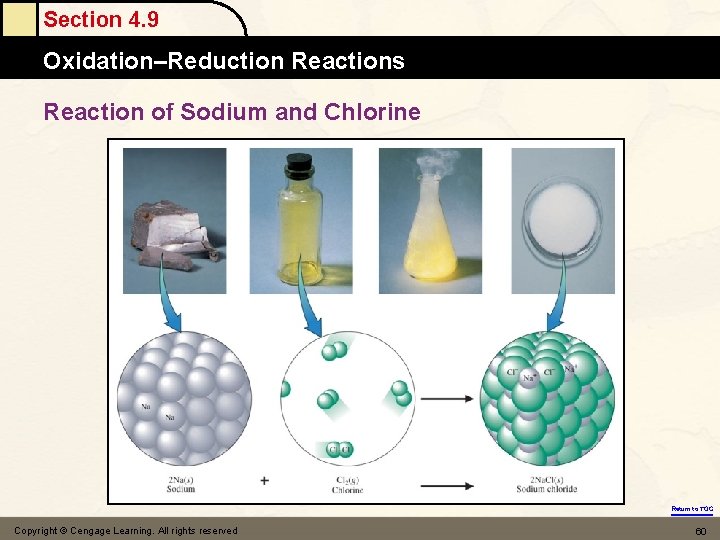
Section 4. 9 Oxidation–Reduction Reactions Reaction of Sodium and Chlorine Return to TOC Copyright © Cengage Learning. All rights reserved 60

Section 4. 9 Oxidation–Reduction Reactions Important rodox reactions • Photosynthesis • The oxidation of sugars, fats, and proteins(in humans) • Combustion reactions Return to TOC Copyright © Cengage Learning. All rights reserved 61

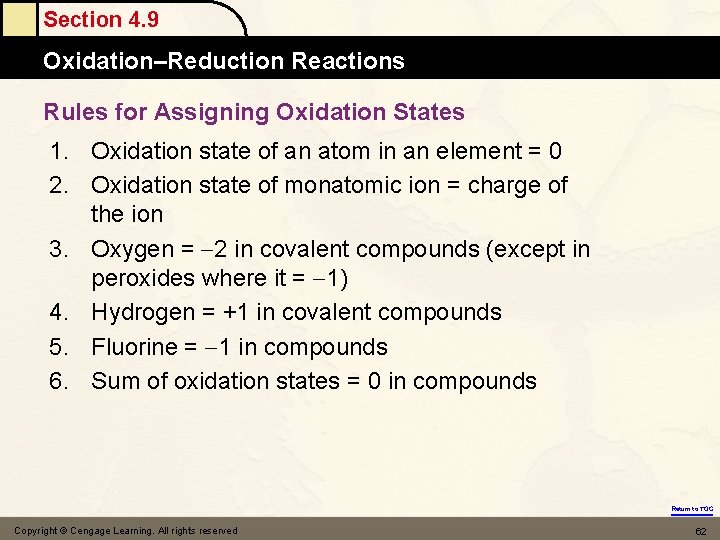
Section 4. 9 Oxidation–Reduction Reactions Rules for Assigning Oxidation States 1. Oxidation state of an atom in an element = 0 2. Oxidation state of monatomic ion = charge of the ion 3. Oxygen = 2 in covalent compounds (except in peroxides where it = 1) 4. Hydrogen = +1 in covalent compounds 5. Fluorine = 1 in compounds 6. Sum of oxidation states = 0 in compounds Return to TOC Copyright © Cengage Learning. All rights reserved 62

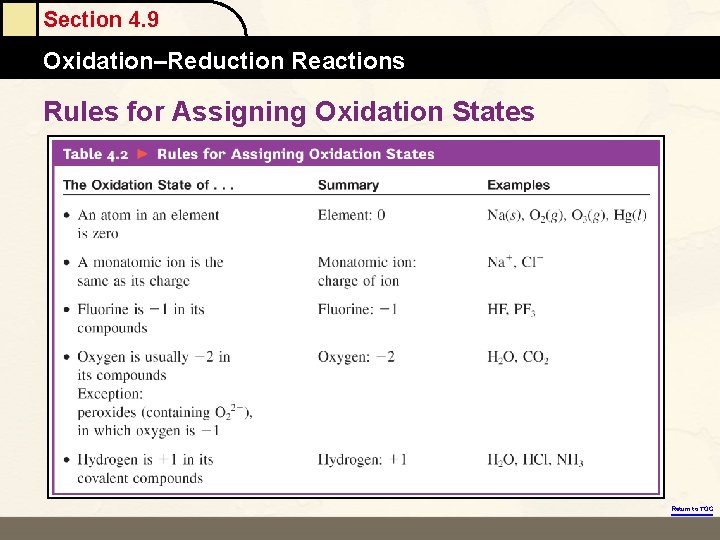
Section 4. 9 Oxidation–Reduction Reactions Rules for Assigning Oxidation States Return to TOC

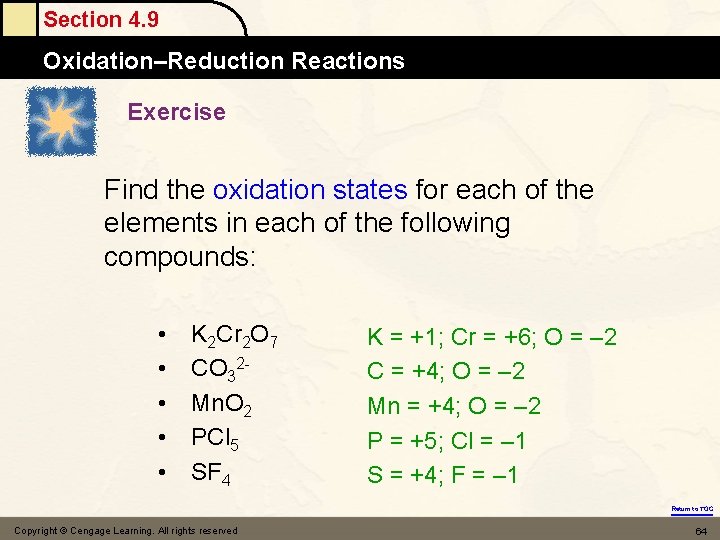
Section 4. 9 Oxidation–Reduction Reactions Exercise Find the oxidation states for each of the elements in each of the following compounds: • • • K 2 Cr 2 O 7 CO 32 Mn. O 2 PCl 5 SF 4 K = +1; Cr = +6; O = – 2 C = +4; O = – 2 Mn = +4; O = – 2 P = +5; Cl = – 1 S = +4; F = – 1 Return to TOC Copyright © Cengage Learning. All rights reserved 64

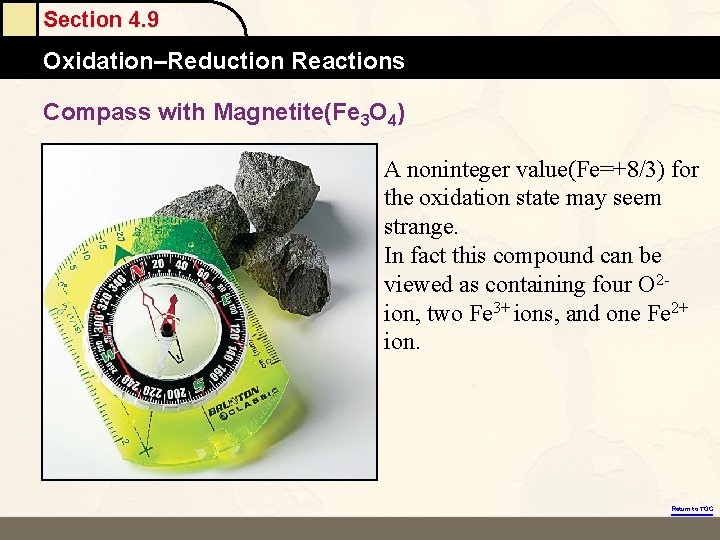
Section 4. 9 Oxidation–Reduction Reactions Compass with Magnetite(Fe 3 O 4) A noninteger value(Fe=+8/3) for the oxidation state may seem strange. In fact this compound can be viewed as containing four O 2 ion, two Fe 3+ ions, and one Fe 2+ ion. Return to TOC

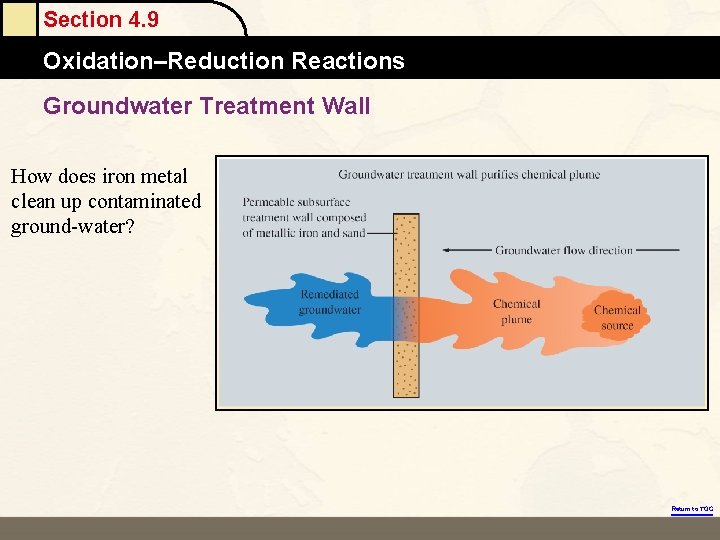
Section 4. 9 Oxidation–Reduction Reactions Groundwater Treatment Wall How does iron metal clean up contaminated ground-water? Return to TOC

Section 4. 9 Oxidation–Reduction Reactions Iron Zeroes in on Pollution Return to TOC Copyright © Cengage Learning. All rights reserved 67

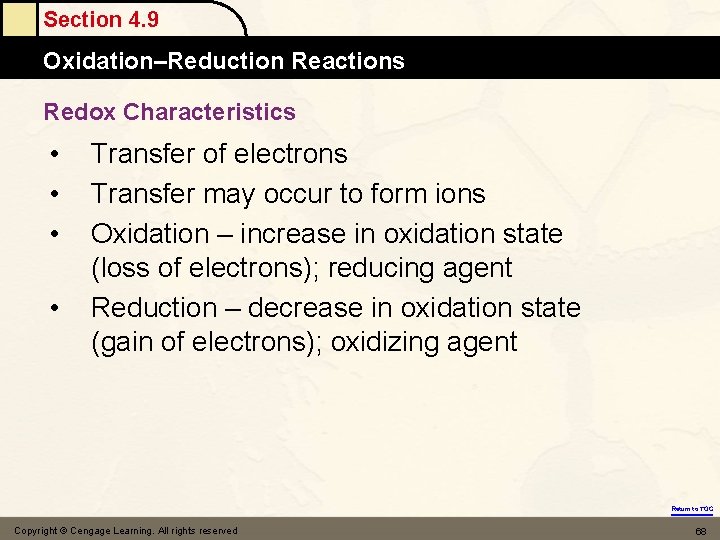
Section 4. 9 Oxidation–Reduction Reactions Redox Characteristics • • Transfer of electrons Transfer may occur to form ions Oxidation – increase in oxidation state (loss of electrons); reducing agent Reduction – decrease in oxidation state (gain of electrons); oxidizing agent Return to TOC Copyright © Cengage Learning. All rights reserved 68

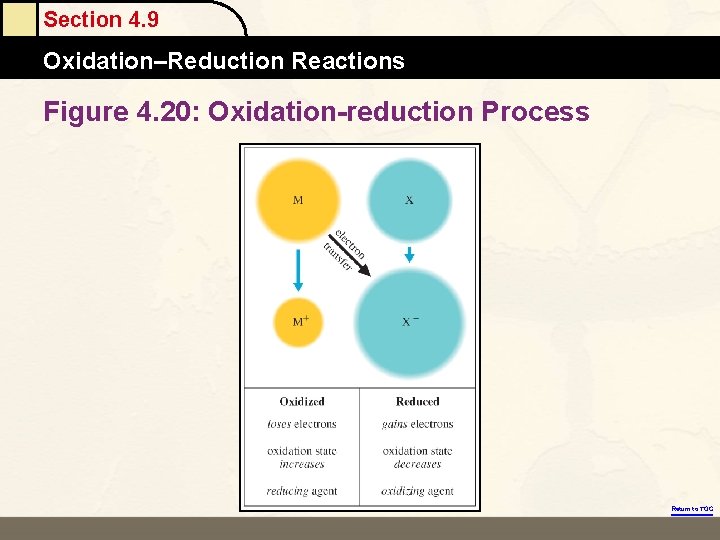
Section 4. 9 Oxidation–Reduction Reactions Figure 4. 20: Oxidation-reduction Process Return to TOC

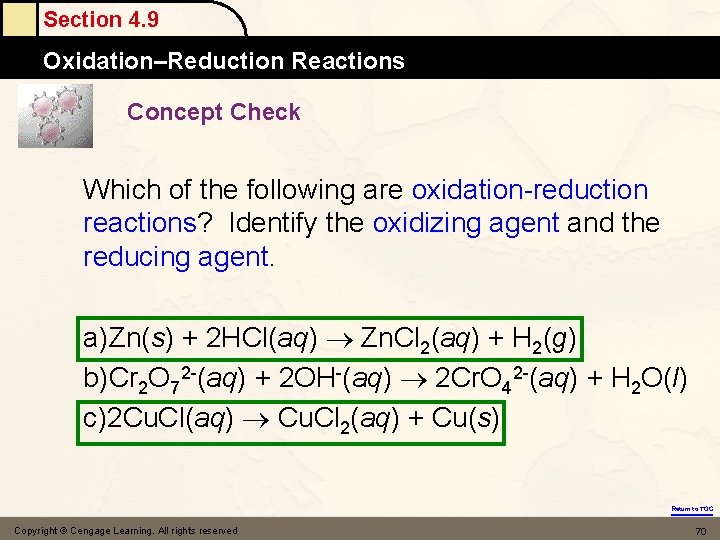
Section 4. 9 Oxidation–Reduction Reactions Concept Check Which of the following are oxidation-reduction reactions? Identify the oxidizing agent and the reducing agent. a)Zn(s) + 2 HCl(aq) Zn. Cl 2(aq) + H 2(g) b)Cr 2 O 72 -(aq) + 2 OH-(aq) 2 Cr. O 42 -(aq) + H 2 O(l) c)2 Cu. Cl(aq) Cu. Cl 2(aq) + Cu(s) Return to TOC Copyright © Cengage Learning. All rights reserved 70

Section 4. 10 Balancing Oxidation–Reduction Equations Balancing Oxidation–Reduction Reactions by Oxidation States 1. Write the unbalanced equation. 2. Determine the oxidation states of all atoms in the reactants and products. 3. Show electrons gained and lost using “tie lines. ” 4. Use coefficients to equalize the electrons gained and lost. 5. Balance the rest of the equation by inspection. 6. Add appropriate states. Return to TOC Copyright © Cengage Learning. All rights reserved 71

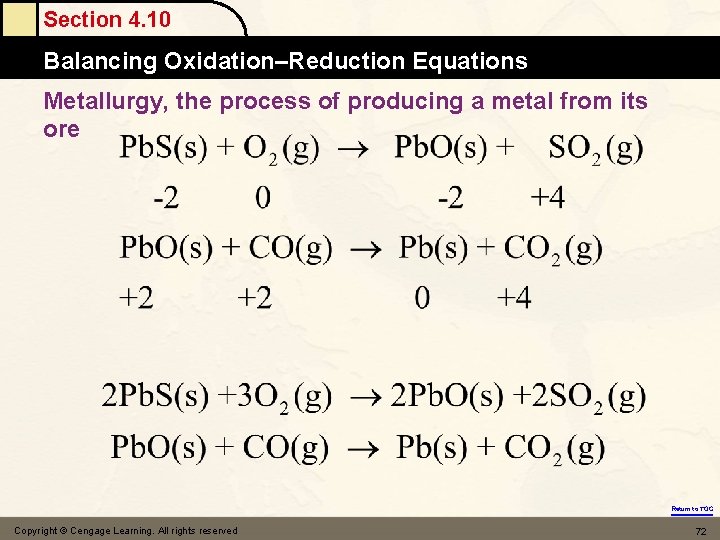
Section 4. 10 Balancing Oxidation–Reduction Equations Metallurgy, the process of producing a metal from its ore Return to TOC Copyright © Cengage Learning. All rights reserved 72

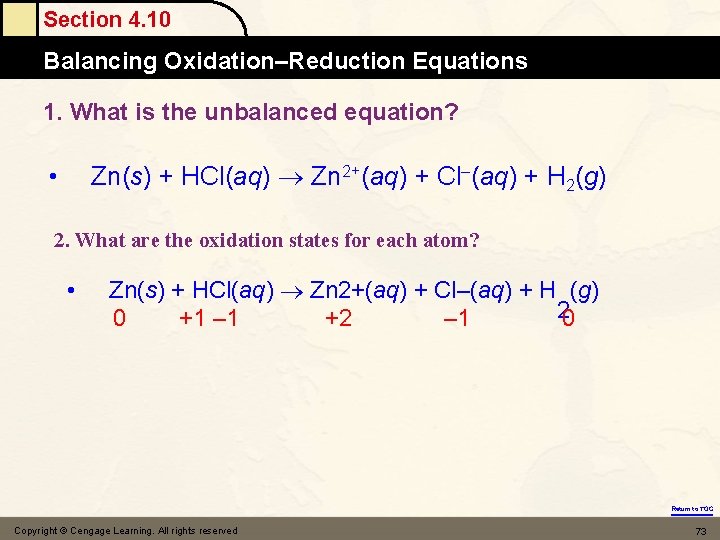
Section 4. 10 Balancing Oxidation–Reduction Equations 1. What is the unbalanced equation? Zn(s) + HCl(aq) Zn 2+(aq) + Cl–(aq) + H 2(g) • 2. What are the oxidation states for each atom? • Zn(s) + HCl(aq) Zn 2+(aq) + Cl–(aq) + H (g) 20 0 +1 – 1 +2 – 1 Return to TOC Copyright © Cengage Learning. All rights reserved 73

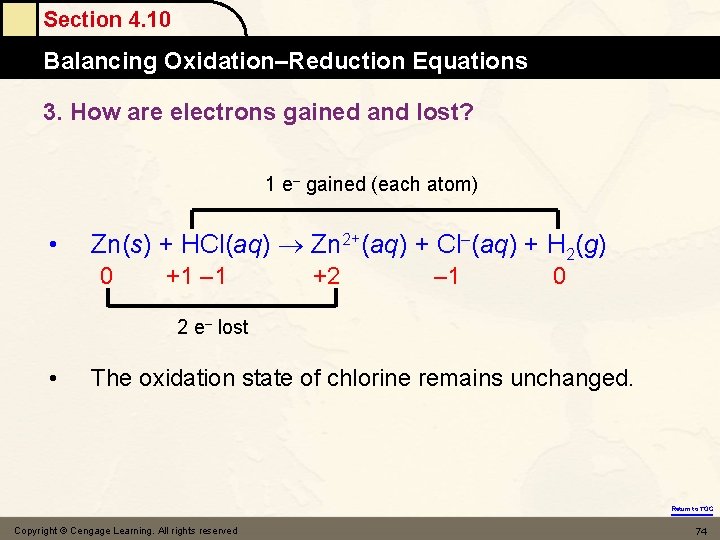
Section 4. 10 Balancing Oxidation–Reduction Equations 3. How are electrons gained and lost? 1 e– gained (each atom) • Zn(s) + HCl(aq) Zn 2+(aq) + Cl–(aq) + H 2(g) 0 +1 – 1 +2 – 1 0 2 e– lost • The oxidation state of chlorine remains unchanged. Return to TOC Copyright © Cengage Learning. All rights reserved 74

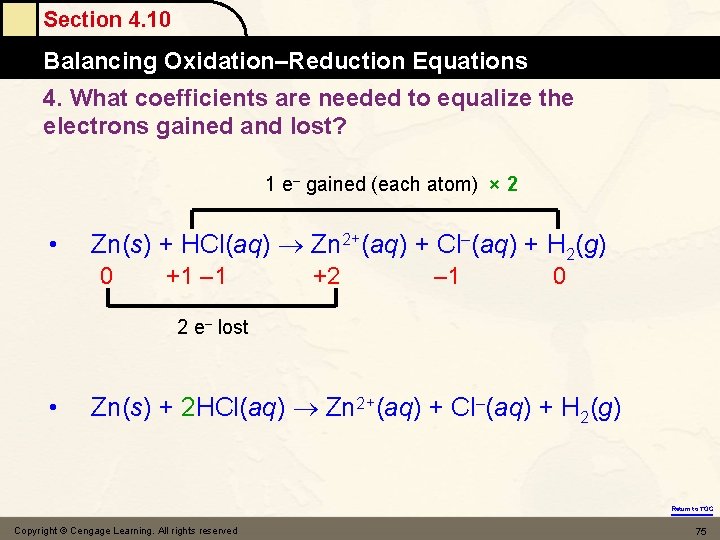
Section 4. 10 Balancing Oxidation–Reduction Equations 4. What coefficients are needed to equalize the electrons gained and lost? 1 e– gained (each atom) × 2 • Zn(s) + HCl(aq) Zn 2+(aq) + Cl–(aq) + H 2(g) 0 +1 – 1 +2 – 1 0 2 e– lost • Zn(s) + 2 HCl(aq) Zn 2+(aq) + Cl–(aq) + H 2(g) Return to TOC Copyright © Cengage Learning. All rights reserved 75

Section 4. 10 Balancing Oxidation–Reduction Equations 5. What coefficients are needed to balance the remaining elements? • Zn(s) + 2 HCl(aq) Zn 2+(aq) + 2 Cl–(aq) + H 2(g) Return to TOC Copyright © Cengage Learning. All rights reserved 76