Chapter 4 The Structure of the Atom 4


















- Slides: 29

Chapter 4: The Structure of the Atom

4. 1 Early Theories of Matter �The idea that matter is composed of tiny particles (which we call atoms) did not even exist a few thousand years ago �The Ancient Greeks (400 BC) believed that everything was made of the 4 fundamental substances: �Fire �Water �Earth �Air �It was thought that each of these four elements could be endlessly divided into smaller and smaller pieces.

Democritus’ Ideas (460 -370 BC) �A Greek who proposed the idea that matter is not infinitely divisible. �He coined the term atomos (atoms) to describe the smallest particles (ultimate particles) �He believed that: �Changes in matter result in changes in grouping of atoms �Matter is composed of empty spaces through which atoms move �Different kinds of atoms have different sizes and shapes �Apparent changes in matter result from changes in the groupings of atoms and not from changes in the atom themselves

�Not all of Democritus’’ ideas agree with modern atomic theory, his belief in the existence of atoms was innovative �Criticism came from Aristotle, Greek philosopher (384 -322 BC) �Aristotle criticized the idea that atoms moved through empty space �He did not believe in empty space “nothingness” �Since Democritus could not defend his theory…his theories were rejected �Because of Aristotle’s influence…he was able to gain wide acceptance for the idea that atoms did not exist �This idea went unchallenged for two thousand years.

�The next ~2000 years are all about Alchemy �Alchemists wanted to turn cheap metals into gold �Robert Boyle(1627 -1697)-”First Chemist” to perform quantitative experiments, measured the relationship between pressure and volume of gases �Antoine Lavoisier(1745 -1794) �Explained nature of combustion �Verified Law of Conservation of Mass �George Stahl (phlogiston) (1660 -1734) �Joseph Priestly (1733 -1804) Discovered Oxygen

John Dalton (1766 -1844) �Was a school teacher in England who revised and revived Democritus’ idea based on scientific research �He developed the Law of Multiple Proportions

Dalton’s Atomic Theory All matter is made up of tiny particles called atoms 2. The atoms of a given element are identical; the atoms of different elements are different in some fundamental way or ways 3. Chemical compounds are formed when atoms of different elements combine with each other. A given compound always has the same relative numbers and types of atoms (Law of Definite Proportions) 1. 4. In a chemical reaction, atoms are separated, combined, or rearranged. Atoms themselves are not changed in a chemical reaction.

Dalton’s Other Ideas �“Matter would be as simple as possible “ �Prepared first table of atomic masses (weights) mostly wrong due to incorrect assumptions �Dalton’s atomic theory not 100% accurate… �It needed to be revised, additional information learned needed to be explained �Atoms are divisible (subatomic particles) �Atoms of given elements do not have identical properties (may have slightly different masses)

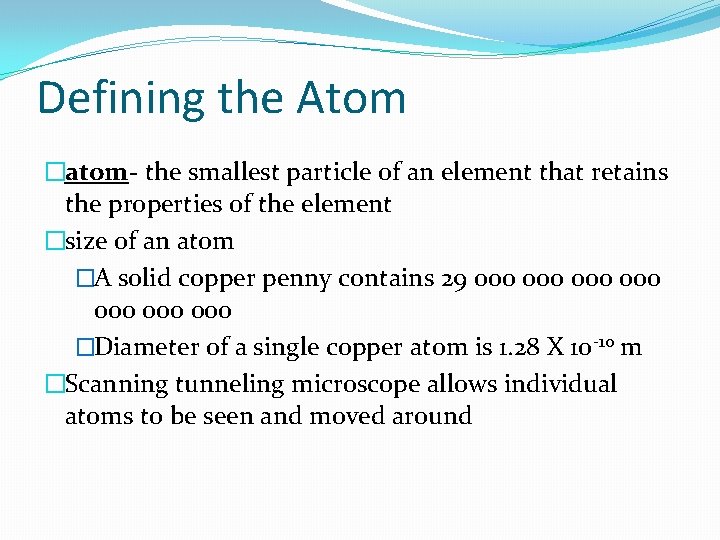
Defining the Atom �atom- the smallest particle of an element that retains the properties of the element �size of an atom �A solid copper penny contains 29 000 000 �Diameter of a single copper atom is 1. 28 X 10 -10 m �Scanning tunneling microscope allows individual atoms to be seen and moved around

�Avogadro- determined at the same temperature and pressure equal volumes of different gases contain the same number of particles (called Avogadro’s Hypothesis) �Led to 22. 4 L = 1 mol of gas and 6. 022 x 1023 molecules = 1 mol @ STP

4. 2 Subatomic Particles and the Nuclear Atom �Discovering the electron �Scientists began to experiment with electricity and look for some sort of relationship between matter and electric charge. They used a vacuum pump (glass tube that most of air (matter) was removed) passed electricity through the glass tube �Working in a dark laboratory, Sir William Crookes (English Physicist) noticed a flash of light within one of the tubes �These rays (radiation) traveled from the cathode (negative terminal of the battery) to the anode (positive terminal of a battery) �Became known as a cathode ray (a ray of radiation that originates from the cathode and travels to the anode of a

Discovering the Electron Con’t �Continued to study cathode rays � cathode ray was a stream of charged particles � the particles carry a negative charge �Because changing the type of electrode or varying the gas (at very low pressure) did not affect the cathode ray production…it was concluded that the ray’s negative particles were found in all forms of matter �electrons- a negatively charged, fast –moving particle with an extremely small mass that is found in all forms of matter and moves through the empty space surrounding an atom’s nucleus.

�William Crookesdeveloped the Cathode-Ray Tube

�American Physicist Robert Millikan (1868 -1953) was then able to determine the electric charge of the electron. �New questions were posed… �knowing matter was neutral and knowing electrons are negatively charged particle �with a very small mass that exist in all matter. What makes up the rest of the mass of an atom? �J. J. Thomson (1856 -1940)-Discovered electrons using the cathode-ray tubes (pg 47) �He proposed a model of the atom known as plum pudding model � looks like chocolate chip cookie dough � did not last long (proved to be incorrect)

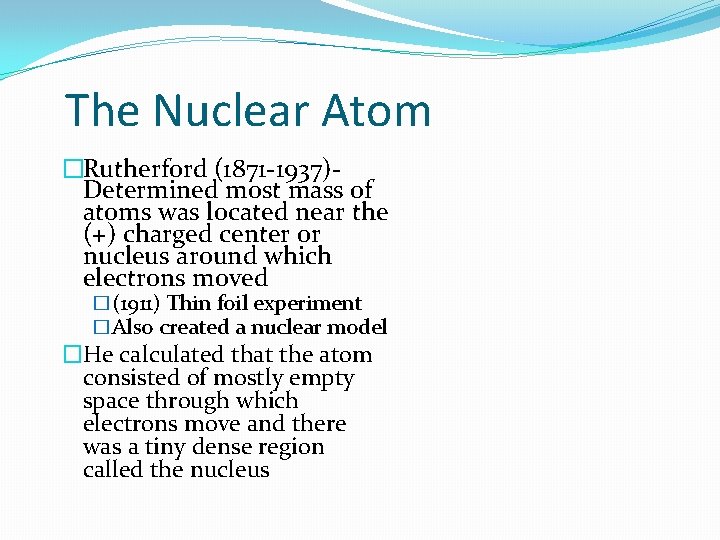
The Nuclear Atom �Rutherford (1871 -1937)Determined most mass of atoms was located near the (+) charged center or nucleus around which electrons moved �(1911) Thin foil experiment �Also created a nuclear model �He calculated that the atom consisted of mostly empty space through which electrons move and there was a tiny dense region called the nucleus

�nucleus- the extremely small positively charged dense center of atom that contains positively charged proton, neutral neutrons and is surrounded by empty space through which one or more negatively charged electrons move. �the nucleus is very small and very dense…it contains most of the mass of the atom �According to Rutherford’s new atomic model (looks like a peach with a pit), most of an atom consists of electrons moving rapidly through empty space surrounded by a dense center called the nucleus

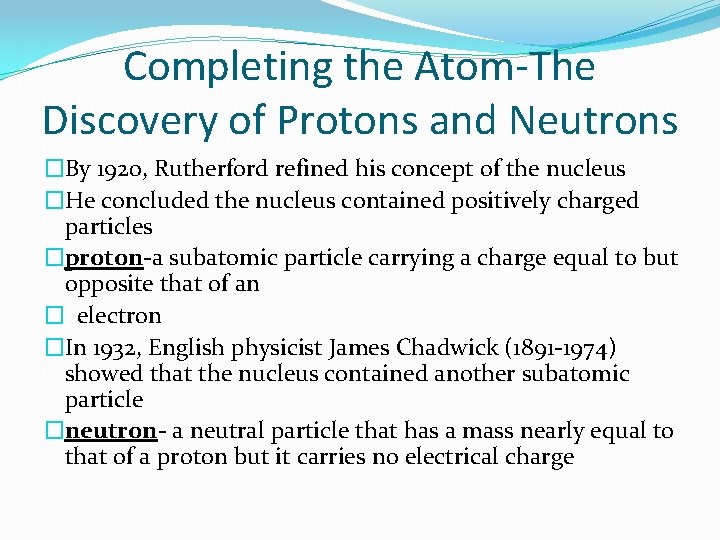
Completing the Atom-The Discovery of Protons and Neutrons �By 1920, Rutherford refined his concept of the nucleus �He concluded the nucleus contained positively charged particles �proton-a subatomic particle carrying a charge equal to but opposite that of an � electron �In 1932, English physicist James Chadwick (1891 -1974) showed that the nucleus contained another subatomic particle �neutron- a neutral particle that has a mass nearly equal to that of a proton but it carries no electrical charge

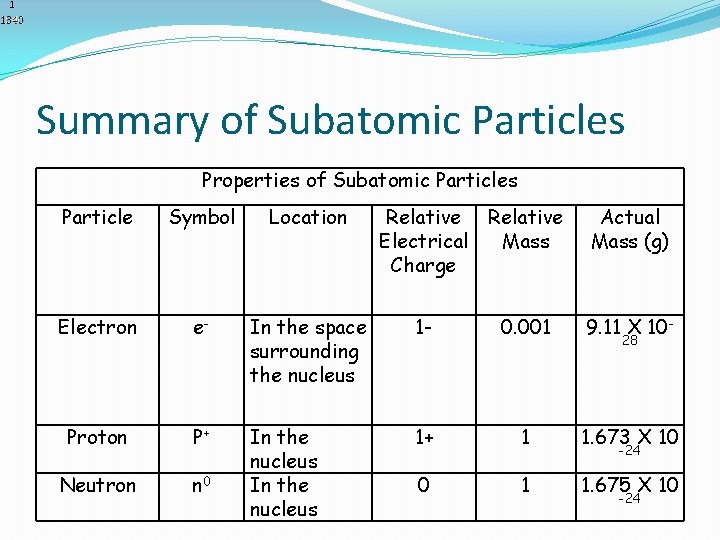
Summary of Subatomic Particles Properties of Subatomic Particles Particle Symbol Location Relative Electrical Mass Charge Actual Mass (g) Electron e- In the space surrounding the nucleus 1 - 0. 001 9. 11 X 10 - Proton P+ 1+ 1 1. 673 X 10 Neutron n 0 In the nucleus 0 1 1. 675 X 10 28 -24

Practice Problem: 1) Select the term in Column B that best matches the phrase in Column A Column B a) Cathode ray 1) electron b) Discovered in 1932 2) proton c) Caused large deflections of alpha 3) neutrons particles in Rutherford’s experiment 4) nucleus d) Has a charge of 1 e) Has no charge f) Contains nearly all of an atom’s mass g) In an atom, the number of these particles is equal to the number of protons h) Identified by Thomson i) Site of an atom’s positive charge j) Has a positive charge and relative mass of 1 k) The center of an atom l) Symbolized by no

4. 3 How Atoms Differ � What makes an atom of one element different from an atom of another element? �differ in the number of subatomic particles �English scientist Henry Moseley (1887 -1915) discovered the atoms of each element contain a unique positive charge in nucleus �Atomic Number-The number of protons in an atom �Atomic number determines the elements position in the periodic table �All atoms are neutral �Atomic Number = Number of protons = Number of electrons

Isotopes and Mass Number �Dalton’s Theory was not 100% correct �wrong about atoms not being divisible (subatomic particles) �wrong about all atoms of a particular element are identical �true that all atoms of a particular element have same number of protons and electrons �the number of neutrons in their nucleus may differ �isotopes-Atoms of the same element with same number of protons, but different number of neutrons � 3 different potassium atoms � all have 19 protons and 19 electrons � one has 20 neutrons � one has 21 neutrons � one has 22 neutrons

�Most elements in nature exist as a mixture of isotopes �Because isotopes differ in neutrons, they differ in mass (since mass of an atom is determined by number of protons and neutrons) �Isotopes containing more neutrons have a greater mass �Isotopes have same chemical behavior �Chemical behavior is determined by the number of electrons not protons or neutrons

�Mass number-the number after an element’s name, representing the sum of its protons and neutrons �potassium-39 (represents potassium atom with 19 protons and 20 neutrons, has mass number of 19+20=39 �use short hand notation to write out isotope �(means mass number 39, atomic number 19, and K=potassium) �(means mass number 40, atomic number 19, and K=potassium) �Number of neutrons=mass number – atomic number

Practice Problems: � 1) One of the four naturally occurring isotopes of chromium has a mass number of 53. Determine the number of protons, electrons, and neutrons in an atom of this isotope and write its symbol. � 2) The other three naturally occurring isotopes of chromium have mass number of 50, 52, and 54. Describe how atoms of these isotopes differ from the isotope mentioned in example 1. � 3) All naturally occurring atoms of fluorine have a mass number of 19. Determine the number of protons, electrons, and neutrons in an atom of fluorine and write the atom’s symbol. � 4) Describe the subatomic particles comprising an isotope of zirconium-94. ) � 5) An atom of a certain element has a mass number of 112 and is known to contain 64 neutrons. Identify the atom and determine the number of electrons and protons the atom contains. � 6) A neutral atom has 78 electrons and a mass number of 198. Identify the atom and determine the number of protons and neutrons in its nucleus.

Mass of Individual Atoms �Atomic mass unit (amu)-scientists have established a standard for the measurement of atomic mass by assigning the carbon-12 atom a mass of 12 atomic mass units (amu) �Atomic mass of an element – is the weighted average mass of the isotopes of that element �It takes into account the mass and abundance of each of the isotopes. �Atomic mass is located on the periodic table (it is the bottom number under element’s name)

Practice Problems: � 1) Copper exists as a mixture of two isotopes. The lighter isotope (Cu-63), with 29 protons and 34 neutrons, makes up 69. 17% of copper atoms. The heavier isotope (Cu-65), with 29 protons and 36 neutrons, constitutes the remaining 30. 83% of copper atoms. The atomic mass of Cu-63 is 62. 930 amu, and the atomic mass of Cu-65 is 64. 928 amu. Use the data above to compute the atomic mass of copper � 2) Gallium occurs in nature as a mixture of two isotopes. They are Ga-69 with a 60. 108% abundance and a mass of 68. 92 amu and Ga-71 with a 39. 892% abundance and an atomic mass of 70. 925. Calculate the atomic mass of gallium. � 3) The following table shows the five isotopes of germanium found in nature, the abundance of each isotope, and the atomic mass of each isotope. Calculate the atomic mass of germanium � Isotope � Abundance (%) and Atomic Mass (amu) � Germanium-70 1. 23 69. 924 � Germanium-72 27. 66 71. 922 � Germanium-73 72. 923 � Germanium-74 35. 94 73. 921 � Germanium-76 7. 44 75. 921 � 4) The element chlorine occurs in nature as a mixture of two isotopes. Chlorine-35 has an atomic mass of 34. 969 amu and makes up 75. 77% of the chlorine atoms. Chlorine-37 atoms make up the remaining 24. 23% of all chlorine. Use the average atomic mass of chlorine from the periodic table to calculate the atomic mass of Cl-37 atoms.

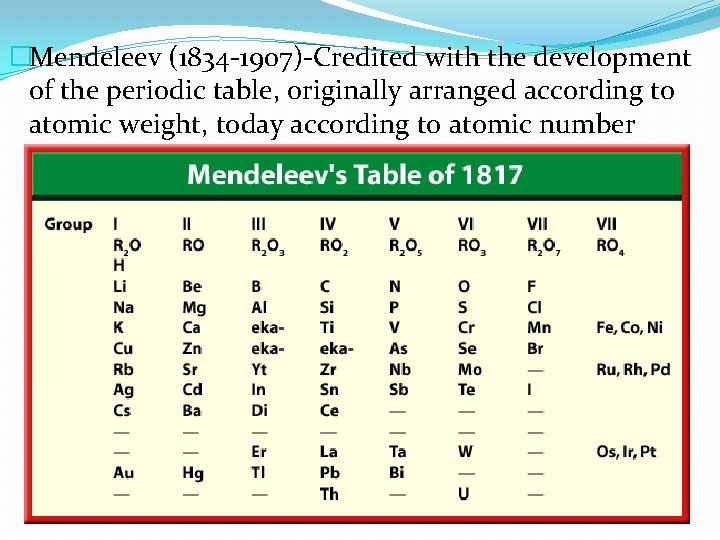
�Mendeleev (1834 -1907)-Credited with the development of the periodic table, originally arranged according to atomic weight, today according to atomic number

�Bohr (1885 -1962)-Electron Cloud Model or Planetary Model

The Quantum Mechanical Model is used as the current atomic model �There is no way to exactly know at any moment of time where an electron is exactly.