Chapter 4 The Periodic Table and Chemical Nomenclature

Chapter 4 The Periodic Table and Chemical Nomenclature Malone and Dolter - Basic Concepts of Chemistry 9 e 1
Setting the Stage – The Periodic Table n n The periodic table is the map of chemical behavior of the elements Chemical nomenclature is the vocabulary of chemistry – how we name compounds and write formulas Malone and Dolter - Basic Concepts of Chemistry 9 e 2

Setting a Goal - Part A Relationships Among the Elements and the Periodic Table n You will be able to explain the significance of the periodic table, its origins, and how different properties of an element can be predicted by its location on the table Malone and Dolter - Basic Concepts of Chemistry 9 e 3

Objective for Section 4 -1 n Describe the origins of the periodic table Malone and Dolter - Basic Concepts of Chemistry 9 e 4

4 -1 The Origin of the Periodic Table n n The elements are grouped in several categories, according to properties and therefore according to position in the periodic table. Two major groupings are metals and nonmetals Malone and Dolter - Basic Concepts of Chemistry 9 e 5

Professor’s Little Joke Malone and Dolter - Basic Concepts of Chemistry 9 e 6
The Origin of the Periodic Table n John Newlands, Dmitri Mendeleev and Lothar Meyer were the main originators of the periodic table of elements Malone and Dolter - Basic Concepts of Chemistry 9 e 7
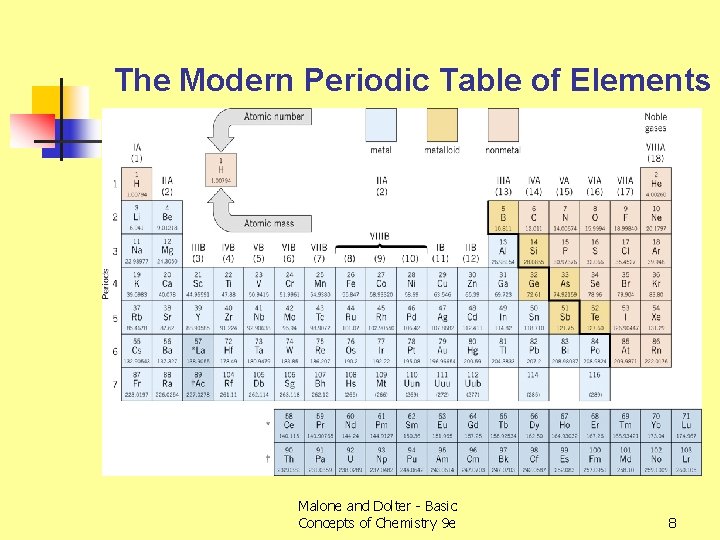
The Modern Periodic Table of Elements Malone and Dolter - Basic Concepts of Chemistry 9 e 8
Metals n n n Tend to form cations in ionic compounds Are ductile (can be drawn into wires) Malleable (can be pounded into sheets) Readily conduct electricity and heat See Figure 4 -1 Malone and Dolter - Basic Concepts of Chemistry 9 e 9

Nonmetals n n n Tend to form anions in ionic compounds Are generally soft solids or gases See Figure 4 -2 Malone and Dolter - Basic Concepts of Chemistry 9 e 10

Classes of metals n Active metals n n n very reactive to air and water include lithium, potassium and sodium Noble metals n n very unreactive gold, silver and copper Malone and Dolter - Basic Concepts of Chemistry 9 e 11

Metalloids n n Have properties intermediate between metals and nonmetals Their most important current use is as semiconductors Malone and Dolter - Basic Concepts of Chemistry 9 e 12

Objective for Section 4 -2 n Using the periodic table, identify a specific element as a metal or a nonmetal and give its period, group number, the name of the group if appropriate, and its physical state Malone and Dolter - Basic Concepts of Chemistry 9 e 13
4. 2 – Using the Periodic Table Locating the main types of elements n n n Metals are found to the left of the periodic table Nonmetals are found to the right of the periodic table Metalloids are located along the metalnonmetal dividing line Malone and Dolter - Basic Concepts of Chemistry 9 e 14
The Periodic Table n n n Elements were originally arranged in columns by chemical properties, then atomic weight The modern periodic table has the elements arranged by atomic number The periodic law - the properties of the elements are periodic (cyclically repeating) functions of their atomic number Malone and Dolter - Basic Concepts of Chemistry 9 e 15
Periodic Divisions n Period n n Horizontal rows in the periodic table Properties of elements across a period change dramatically Each period ends with a member of the noble gas family Group n n Vertical columns in the periodic table Properties of elements in the same group are similar Malone and Dolter - Basic Concepts of Chemistry 9 e 16

Main Group Elements n n n Group IA - alkali metals Group IIA - alkaline earths Group VIA - chalcogens Group VIIA - halogens Group VIIIA - noble gases * These are the groups with specific names Malone and Dolter - Basic Concepts of Chemistry 9 e 17
Transition Elements n Group B elements n n Metals with multiple oxidation states Includes noble or coinage metals (Cu, Ag, Au) Platinum group metals (Ru, Rh, Pd, Os, Ir, Pt) Structural metals such as Fe and Cr Malone and Dolter - Basic Concepts of Chemistry 9 e 18
Inner transition elements n Lanthanides n n n Often called rare earth elements due to the difficulty in isolating pure samples Chemical properties almost identical Actinides n Radioactive elements, many synthetic Malone and Dolter - Basic Concepts of Chemistry 9 e 19
Physical States of Elements n n n Reference condition is 1 atmosphere of pressure and 25 °C Gaseous elements - H 2, N 2, O 2, F 2, Cl 2, noble gases Liquid elements - Hg, Br 2 Others? All other elements are solids Other molecular nonmetals are not diatomic and may have several forms (allotropes) Malone and Dolter - Basic Concepts of Chemistry 9 e 20
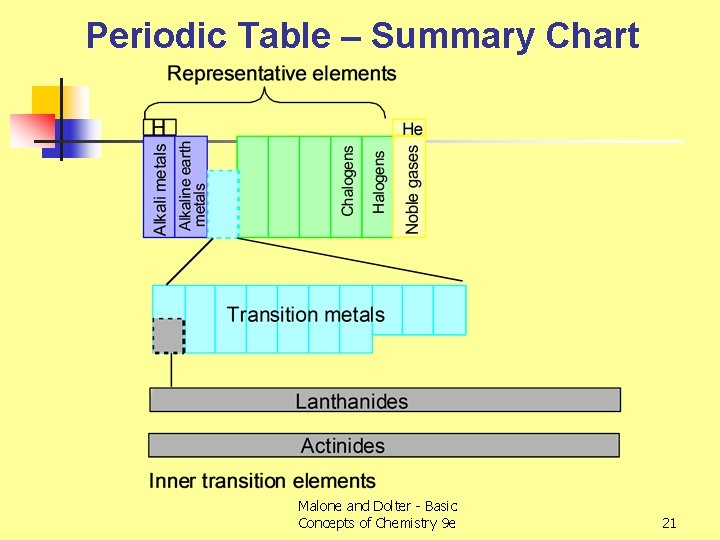
Periodic Table – Summary Chart Malone and Dolter - Basic Concepts of Chemistry 9 e 21

Setting a Goal – Part B The Formulas and Names of Compounds n You will learn how to systematically name various types of molecular and ionic compounds and determine the formulas of compounds from the names Malone and Dolter - Basic Concepts of Chemistry 9 e 22

Objective for Section 4 -3 n Write and name ionic compounds involving a metal and a nonmetal using IUPAC conventions Malone and Dolter - Basic Concepts of Chemistry 9 e 23

A Little Joke on Nomenclature A research chemist walked into a pharmacy and asks, “Do you have (5 a, 6 a)-7, 8 -didehydro-4, 5 epoxy-3 -methoxy-17 -methylmorphinan-6 -ol? ” The pharmacist scratched her head and said, “Do you mean codeine? ” “That’s it!”, said the chemist, “I can never remember that word!” Malone and Dolter - Basic Concepts of Chemistry 9 e 24

4 -3 Naming and Writing Formulas of Metal-Nonmetal Binary Compounds n n Binary compounds are composed of two different elements Two types of metal-nonmetal binary compounds n n Metals exhibiting only one oxidation state forming a compound with a nonmetal Metals exhibiting two or more oxidation states forming a compound with a nonmetal Malone and Dolter - Basic Concepts of Chemistry 9 e 25

Metals with only one Oxidation State n Groups of metals with only one common oxidation state n n n alkali metals +1 alkaline earths +2 Zn +2 Al +3 All other metals can exhibit more that one oxidation state Malone and Dolter - Basic Concepts of Chemistry 9 e 26

Anions in Negative Oxidation States n Nonmetallic anions usually exhibit one negative oxidation state n n Halogens -1 Chalcogens -2 N, P -3 C -4 Malone and Dolter - Basic Concepts of Chemistry 9 e 27

Forming Ionic Compounds n n n Metal and nonmetal combine to neutralize charge Such compounds are often termed salts Individual anions and cations do not exist separately – always found together Malone and Dolter - Basic Concepts of Chemistry 9 e 28
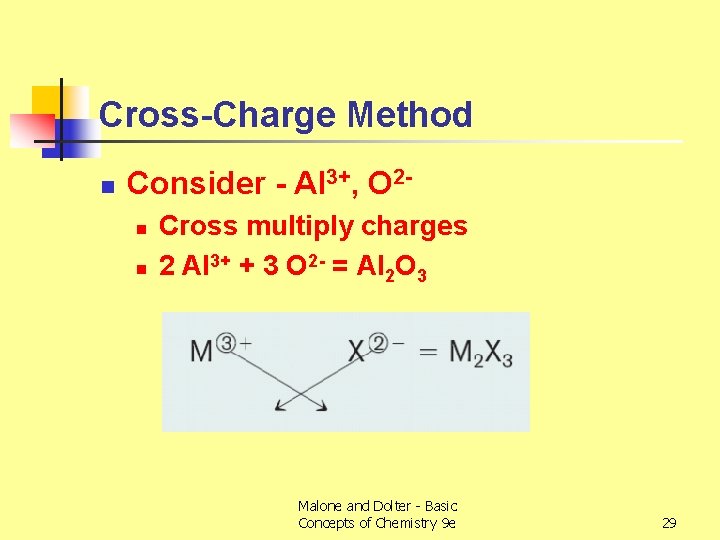
Cross-Charge Method n Consider - Al 3+, O 2 n n Cross multiply charges 2 Al 3+ + 3 O 2 - = Al 2 O 3 Malone and Dolter - Basic Concepts of Chemistry 9 e 29

Naming Binary Compounds n n n Use name of metal with no changes Change the name of the anion by taking the “stem” and add the suffix ide Examples n n Na. Cl - sodium chloride Mg. Cl 2 - magnesium chloride Malone and Dolter - Basic Concepts of Chemistry 9 e 30
Metals with Multiple Oxidation States n n Two systems: Stock and “Classical” Stock system n n Metal name and the oxidation state in Roman numbers in parentheses Fe 2+ = iron(II) Form compound by balancing charge of metal with correct number of nonmetals Co. Cl 3 = cobalt(III) chloride Malone and Dolter - Basic Concepts of Chemistry 9 e 31
Classical Nomenclature n n Metals in multiple oxidation states usually have one or two common oxidation states First row transition metals are +2 and +3 (except Cu 2+ and Cu+) Use -ous suffix for lower common oxidation state Use -ic suffix for higher common oxidation state Malone and Dolter - Basic Concepts of Chemistry 9 e 32

Examples n n n Co. Cl 3 - cobaltic chloride Ni. Cl 2 - nickelous chloride For metals with Latin names, use the Latin names Cu. Cl - cuprous chloride Fe. Br 3 - ferric bromide Malone and Dolter - Basic Concepts of Chemistry 9 e 33

Objective for Section 4 -4 n Write and name compounds containing polyatomic ions using IUPAC conventions Malone and Dolter - Basic Concepts of Chemistry 9 e 34

4 -4 Naming and Writing Formulas of Compounds with Polyatomic Ions n n n Polyatomic ions (listed in Table 4 -2) act as monoatomic ions in most circumstances Most polyatomic ions are oxyanions, but some are simply polyatomic species with trivial names Treat the polyatomic ion as a monoatomic ion in the cross-charge method formula Malone and Dolter - Basic Concepts of Chemistry 9 e 35

Oxyanions n n n Anions composed of oxygen and another element Other elements can be a metal or a nonmetal Examples n SO 42 -, NO 2 -, PO 43 -, Mn. O 4 -, Cr. O 42 - Malone and Dolter - Basic Concepts of Chemistry 9 e 36

Naming Oxyanions n Need common oxidation states n n n most common oxidation state for nonmetals is the group number (except for the halogens) next most common oxidation state is the group number minus one Use -ate suffix for higher oxidation state and -ite suffix for next higher oxidation state Malone and Dolter - Basic Concepts of Chemistry 9 e 37

Examples n n n SO 42 - - sulfate SO 32 - - sulfite NO 3 - - nitrate NO 2 - - nitrite Salts with these oxyanions n n Na 2 SO 4 - sodium sulfate KNO 3 - potassium nitrate Malone and Dolter - Basic Concepts of Chemistry 9 e 38

Salts n n n Compounds formed by combining a cation and an anion in the proper ratio to yield a neutral species Examples include Na. Cl, K 2 SO 4, NH 4 I Often formed by the reaction of a acid containing the anion and a hydroxide compound containing the cation Malone and Dolter - Basic Concepts of Chemistry 9 e 39

Examples of Salt Formation Occurring out of Water 2 Na + Cl 2 2 Na. Cl NH 3 + HCl Malone and Dolter - Basic Concepts of Chemistry 9 e NH 4 Cl 40

Objective for Section 4 -5 n Name binary molecular (nonmetal) compounds using proper Greek prefixes Malone and Dolter - Basic Concepts of Chemistry 9 e 41

4 -5 Naming Nonmetal-Nonmetal Binary Compounds n n Name nonmetal further to the left of the periodic table first with no changes Name nonmetal further to the right of the periodic table second with the -ide suffix Use Greek prefixes to indicate the number of each one Stock system is rarely used in this case since it can yield ambiguous results Malone and Dolter - Basic Concepts of Chemistry 9 e 42
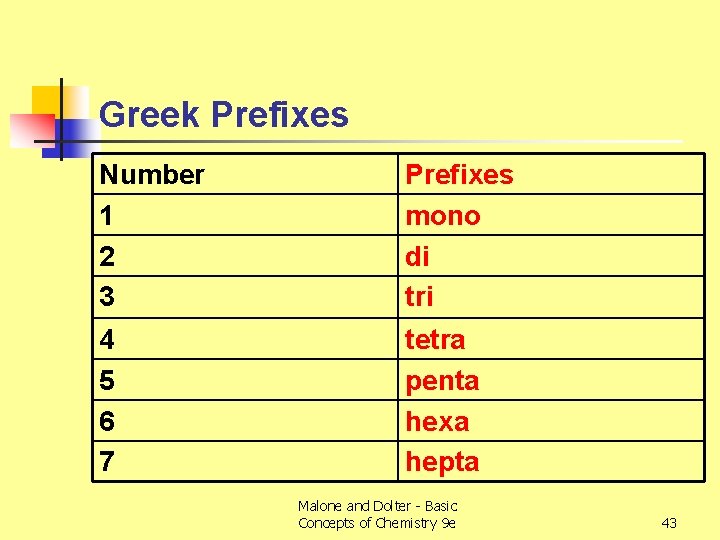
Greek Prefixes Number 1 2 3 Prefixes mono di tri 4 5 6 7 tetra penta hexa hepta Malone and Dolter - Basic Concepts of Chemistry 9 e 43

Examples n n n N 2 O 3 – dinitrogen trioxide N 2 O 5 – dinitrogen pentoxide CO 2 – carbon dioxide P 2 O 5 – diphosphorus pentoxide C 3 O 2 – tricarbon dioxide (carbon suboxide) CO – carbon monoxide Malone and Dolter - Basic Concepts of Chemistry 9 e 44

Objective for Section 4 -6 n Name and write the formulas of acids Malone and Dolter - Basic Concepts of Chemistry 9 e 45

4 -6 Naming Acids n Binary acids n n Name begins with hydro Then add stem of nonmetal plus -ic End the name with acid Examples n n HCl - hydrochloric acid H 2 S - hydrosulfuric acid Malone and Dolter - Basic Concepts of Chemistry 9 e 46

Oxyacids (see Table 4 -6) n Take oxyanion suffix and convert n n Change -ate to -ic Change -ite to -ous Do not use hydro- in the beginning Examples n n H 2 SO 4 - sulfuric acid H 2 SO 3 - sulfurous acid Malone and Dolter - Basic Concepts of Chemistry 9 e 47
- Slides: 47