Chapter 4 The Periodic Table 4 1 History

Chapter 4: The Periodic Table

4. 1 History of the Periodic Table Objectives: (1)To describe the historical development of the periodic table. (2)To describe the organization of the modern periodic table according to the periodic law.
John Newlands (1837 -1898) • Used the properties of elements to sort them into groups and also by increasing atomic mass. • Noticed that the properties repeated every 8 elements. • He called this repeating pattern the Law of Octaves
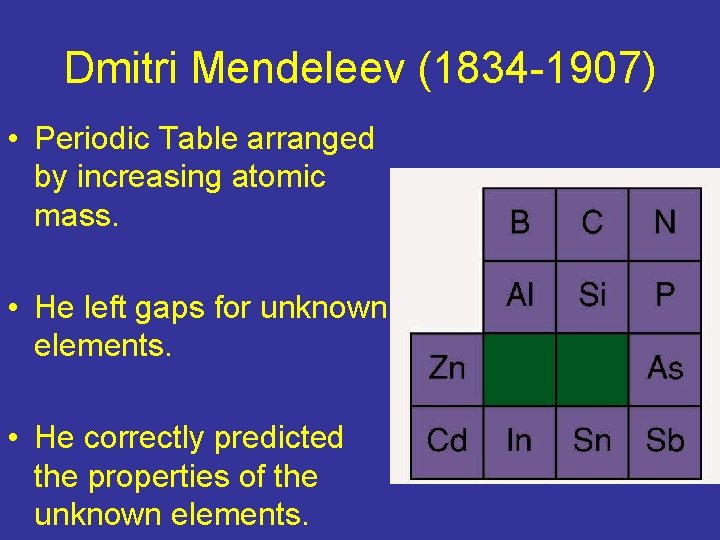
Dmitri Mendeleev (1834 -1907) • Periodic Table arranged by increasing atomic mass. • He left gaps for unknown elements. • He correctly predicted the properties of the unknown elements.
Dmitri Mendeleev (1834 -1907) • Observed that elements in his table do not always increase by atomic mass. • Example: Tellurium (Te) and Iodine (I) • His periodic table was the first periodic table accepted by other scientist.

Henry Moseley • Arranged the periodic table by atomic number.
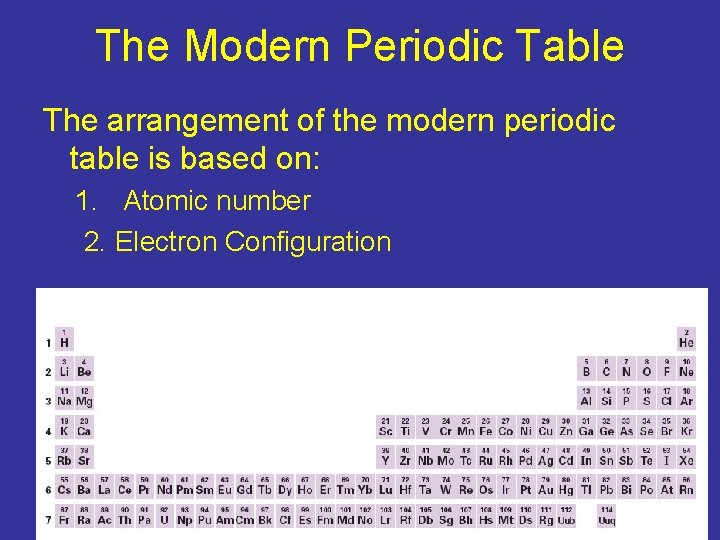
The Modern Periodic Table The arrangement of the modern periodic table is based on: 1. Atomic number 2. Electron Configuration

The Periodic Law • The Periodic Law: The repeating physical and chemical properties of elements change periodically with their atomic number.
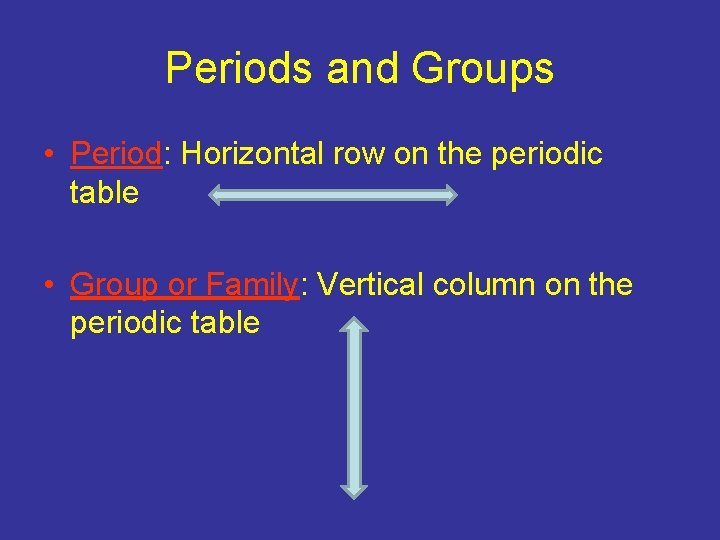
Periods and Groups • Period: Horizontal row on the periodic table • Group or Family: Vertical column on the periodic table

Valence Electrons • Elements in the same period have the same amount of occupied energy levels. • Elements in the same group have the same amount of valence electrons. • Valence electrons: electrons in the outermost shell
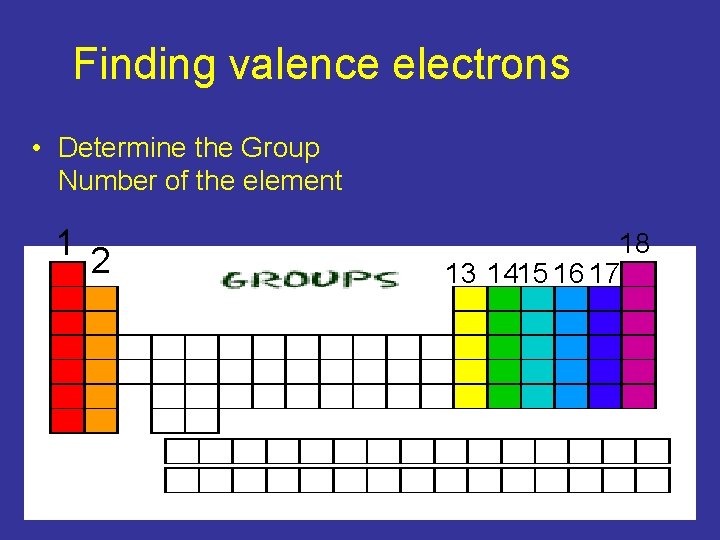
Finding valence electrons • Determine the Group Number of the element 1 2 18 13 1415 16 17

Determining Valence electrons • Groups 1 and 2 have 1 and 2 valence electrons respectively • What group is Calcium part of ? • 2 • How many Valence electrons does Calcium have? 2

Groups 13 -18 • Any of the groups 13 -18 we must only look at the back half of the group number 1 3 or subtract 10 from the groups number to find the number of valence electrons. 13 – 10 = 3

• • • What group does Sulfur belong to? 16 How many valence electrons does it have? 6 What group does Aluminum belong to? 13 How many valence electrons does it have? 3 What group does Krypton belong to? 18 How many valence electrons does it have? 8

Metals, Nonmetals, and Metalloids • One way to classify elements in the periodic table is as metals, nonmetals, and metalloids. • Moving across a period, the properties of elements become less metallic and more nonmetallic.
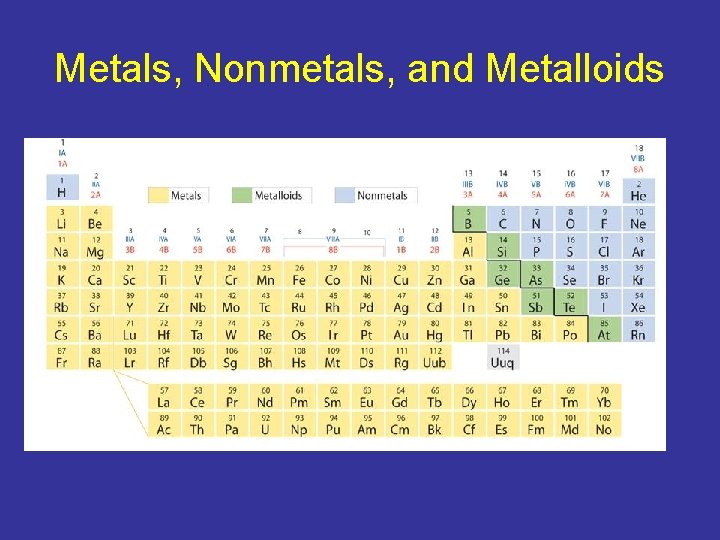
Metals, Nonmetals, and Metalloids
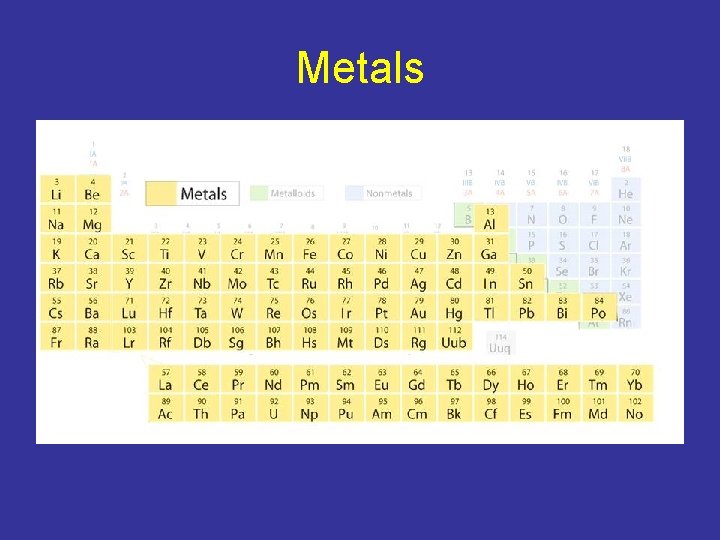
Metals

Metals -Elements that are solids at room temperature (except for Hg). - Good conductors of heat and electric current. - 80% of elements are metals. - Metals have a high luster, are ductile, and are malleable. - Luster: Shiny - Ductile: Can be drawn into wires - Malleable: Can be hammered into thin sheets without breaking.

Metals Uses of Iron, Copper, and Aluminum

Metals

Metals
Nonmetals
Nonmetals • Nonmetals are poor conductors of heat and electricity. • Nonmetals are brittle – Brittle: Will shatter if hit with a hammer. • Most nonmetals are gases at room temperature. – Sulfur and Phosphorus are solids – Bromine is a dark-red liquid.
Metalloids

Metalloids • Metalloids have chemical and physical properties are similar to those of metals and nonmetals. • Some are semiconductors, such as Si and Ge.

Practice • Identify the following as metals, nonmetals, or metalloids: 1. Li 2. Ti 3. Br 4. Si 5. As 6. Kr 7. Fe

Practice • Identify the following as metals, nonmetals, or metalloids: 1. Li: Metal 2. Ti: Metal 3. Br: Nonmetal 4. Si: Metalloid 5. As: Metalloid 6. Kr: Nonmetal 7. Fe: Metal
- Slides: 27