Chapter 4 The Electromagnetic Spectrum Part 1 Visible

Chapter 4 The Electromagnetic Spectrum Part 1
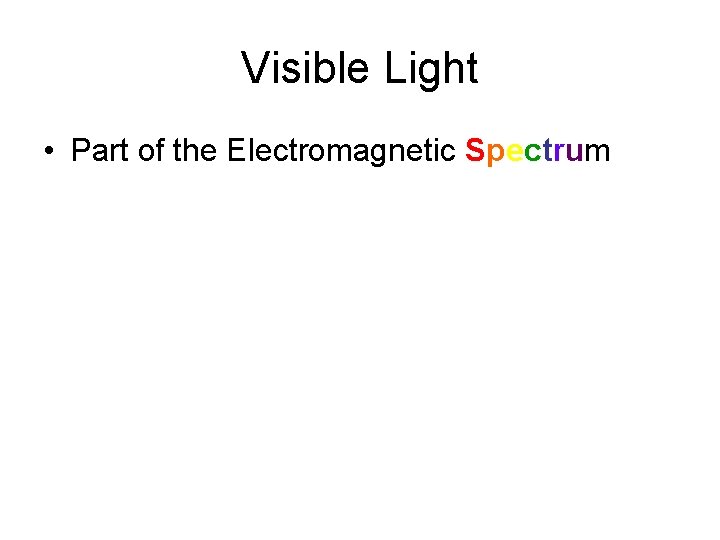
Visible Light • Part of the Electromagnetic Spectrum
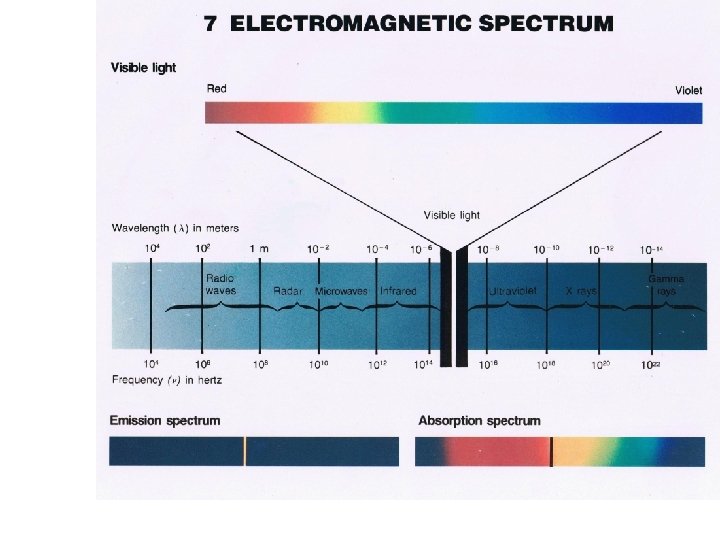
All types of electromagnetic energy move in waves. • Light and sound move by waves. • Light moves at 3 x 108 m/s • Sound moves at 340 m/s • 1 mile = 1609 m
All types of electromagnetic energy move in waves. • Light moves at 3 x 108 m/s • Sound moves at 340 m/s • Is sound a form of electromagnetic radiation?
Electromagnetic Waves • Properties of waves include speed, frequency and wavelength • All electromagnetic waves including light travel at a speed of 3 x 108 m/s.
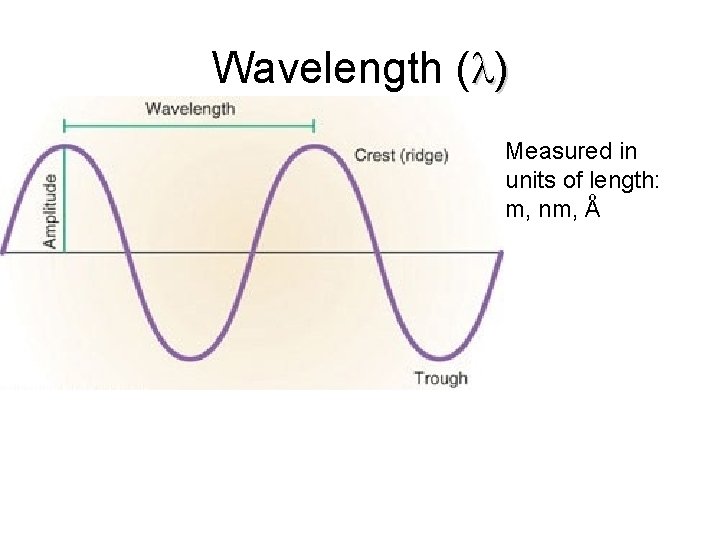
Wavelength ( ) Measured in units of length: m, nm, Aº
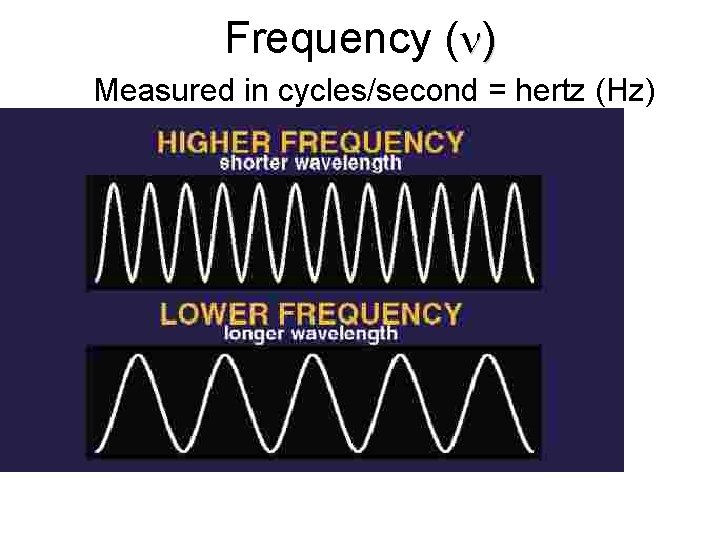
Frequency ( ) Measured in cycles/second = hertz (Hz)
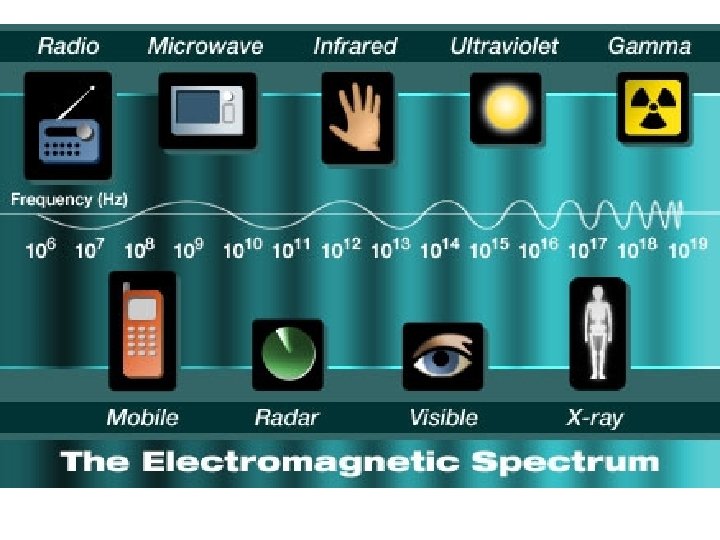
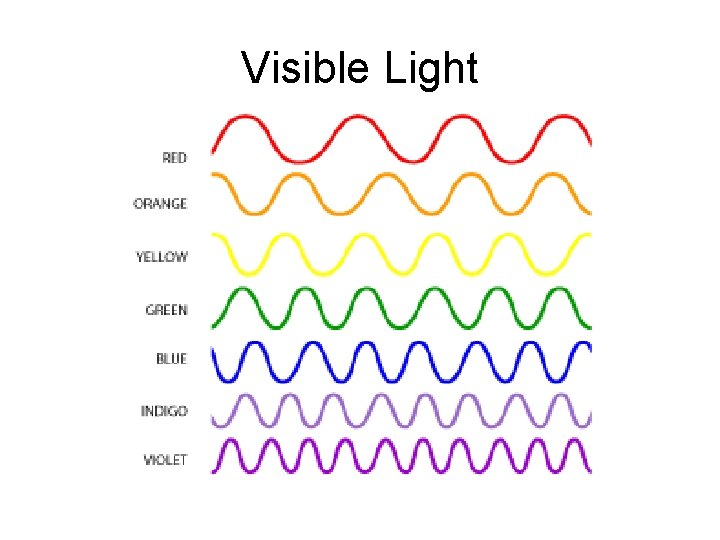
Visible Light

Why do we want to understand electromagnetic radiation (light)? • Well, for one thing, it may someday allow us to become invisible.
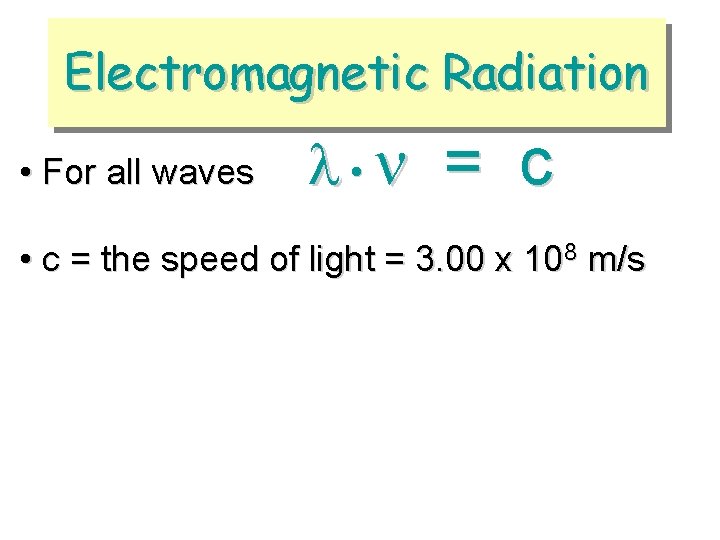
Electromagnetic Radiation • For all waves • = c • c = the speed of light = 3. 00 x 108 m/s

A photon of red light has a wavelength of 665 nm. What is the frequency of this light?

A photon of red light has a wavelength of 665 nm. What is the frequency of this light? • 665 nm = 665 x 10 -9 m

A photon of red light has a wavelength of 665 nm. What is the frequency of this light? • 665 nm = 665 x 10 -9 m • c= •

A photon of red light has a wavelength of 665 nm. What is the frequency of this light? • 665 nm = 665 x 10 -9 m • c= • • = c ÷ = 3. 00 x 108 m/s ÷ 665 x 10 -9 m

A photon of red light has a wavelength of 665 nm. What is the frequency of this light? • • 665 nm = 665 x 10 -9 m c= • = c ÷ = 3. 00 x 108 m/s ÷ 665 x 10 -9 m = 4. 511278 x 1014/s or Hz

A photon of red light has a wavelength of 665 nm. What is the frequency of this light? • • • 665 nm = 665 x 10 -9 m c= • = c ÷ = 3. 00 x 108 m/s ÷ 665 x 10 -9 m = 4. 511278 x 1014/s or Hz = 4. 51 x 1014/s or Hz

An x-ray has a frequency of 7. 25 x 1020 Hz. What is the wavelength?

An x-ray has a frequency of 7. 25 x 1020 Hz. What is the wavelength? • 7. 25 x 1020 Hz = 7. 25 x 1020/s

An x-ray has a frequency of 7. 25 x 1020 Hz. What is the wavelength? • 7. 25 x 1020 Hz = 7. 25 x 1020/s • c= •

An x-ray has a frequency of 7. 25 x 1020 Hz. What is the wavelength? • 7. 25 x 1020 Hz = 7. 25 x 1020/s • c= • • = c ÷ = 3. 00 x 108 m/s ÷ 7. 25 x 1020/s

An x-ray has a frequency of 7. 25 x 1020 Hz. What is the wavelength? • • 7. 25 x 1020 Hz = 7. 25 x 1020/s c= • = c ÷ = 3. 00 x 108 m/s ÷ 7. 25 x 1020/s = 4. 137931 x 10 -13 m

An x-ray has a frequency of 7. 25 x 1020 Hz. What is the wavelength? • • • 7. 25 x 1020 Hz = 7. 25 x 1020/s c= • = c ÷ = 3. 00 x 108 m/s ÷ 7. 25 x 1020/s = 4. 137931 x 10 -13 m = 4. 14 x 10 -13 m

Energy of Waves
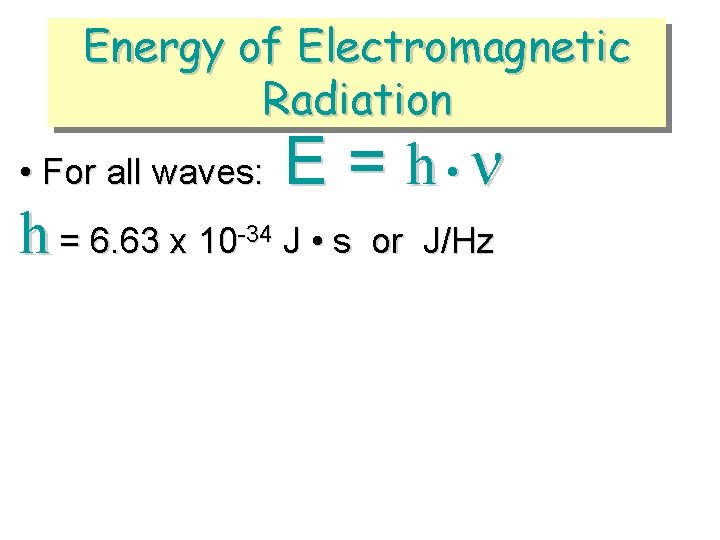
Energy of Electromagnetic Radiation • For all waves: h = 6. 63 x 10 -34 E = h • J • s or J/Hz

A photon of red light has a wavelength of 665 nm. What is the energy of this light?

A photon of red light has a wavelength of 665 nm. What is the energy of this light? • • • 665 nm = 665 x 10 -9 m c= • = c ÷ = 3. 00 x 108 m/s ÷ 665 x 10 -9 m = 4. 511278 x 1014/s or Hz = 4. 51 x 1014/s or Hz

A photon of red light has a wavelength of 665 nm. What is the energy of this light? • • • 665 nm = 665 x 10 -9 m c= • = c ÷ = 3. 00 x 108 m/s ÷ 665 x 10 -9 m = 4. 511278 x 1014/s or Hz = 4. 51 x 1014/s or Hz E = h • = (6. 63 x 10 -34 J • s)(4. 51 x 1014/s)

A photon of red light has a wavelength of 665 nm. What is the energy of this light? • • 665 nm = 665 x 10 -9 m c= • = c ÷ = 3. 00 x 108 m/s ÷ 665 x 10 -9 m = 4. 511278 x 1014/s or Hz = 4. 51 x 1014/s or Hz E = h • = (6. 63 x 10 -34 J • s)(4. 51 x 1014/s) E = 2. 99013 x 10 -19 J

A photon of red light has a wavelength of 665 nm. What is the energy of this light? • • 665 nm = 665 x 10 -9 m c= • = c ÷ = 3. 00 x 108 m/s ÷ 665 x 10 -9 m = 4. 511278 x 1014/s or Hz = 4. 51 x 1014/s or Hz E = h • = (6. 63 x 10 -34 J • s)(4. 51 x 1014/s) E = 2. 99013 x 10 -19 J = 2. 99 x 10 -19 J
An x-ray has a frequency of 7. 25 x 1020 Hz. What is its energy?

An x-ray has a frequency of 7. 25 x 1020 Hz. What is it’s energy? • E = h • = (6. 63 x 10 -34 J/Hz)(7. 25 x 1020 Hz)

An x-ray has a frequency of 7. 25 x 1020 Hz. What is it’s energy? • E = h • = (6. 63 x 10 -34 J/Hz)(7. 25 x 1020 Hz) • E = 4. 80675 x 10 -13 J

An x-ray has a frequency of 7. 25 x 1020 Hz. What is it’s energy? • E = h • = (6. 63 x 10 -34 J/Hz)(7. 25 x 1020 Hz) • E = 4. 80675 x 10 -13 J • E = 4. 81 x 10 -13 J
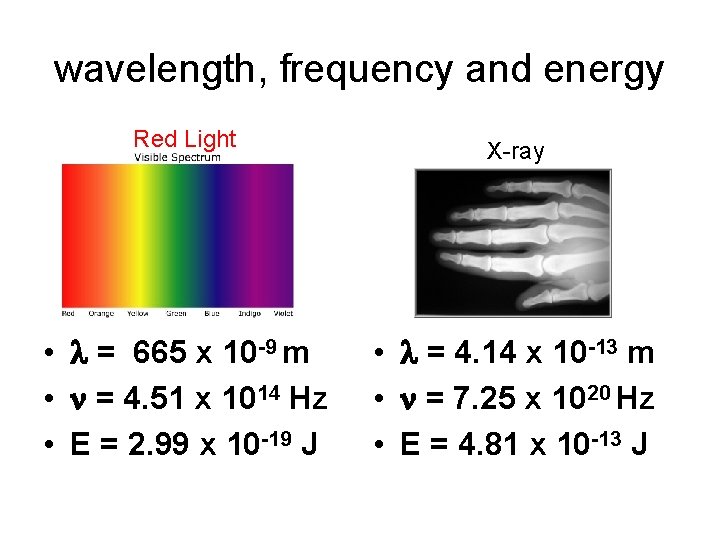
wavelength, frequency and energy Red Light • = 665 x 10 -9 m • = 4. 51 x 1014 Hz • E = 2. 99 x 10 -19 J X-ray • = 4. 14 x 10 -13 m • = 7. 25 x 1020 Hz • E = 4. 81 x 10 -13 J

Wavelength, frequency and energy • Wavelength and frequency have an indirect relationship. • Energy and frequency have a direct relationship. • Electromagnetic radiation with a shorter wavelength will have a higher frequency and higher energy. • Electromagnetic radiation with a longer wavelength will have a lower frequency and lower energy.
- Slides: 37