Chapter 4 The Development of The Atomic Theory









- Slides: 20

Chapter 4 The Development of The Atomic Theory

The Beginning • The first theory of atoms was proposed more than 2, 000 years ago (460 B. C. ) • The Greek philosopher Democritus suggested that the universe was made of indivisible units. • He would soon call the indivisible units atoms.

Democritus • Democritus proposed 6 points to his theory of atoms: • 1. All matter is composed of atoms, which are bits of matter too small to be seen. These atoms CANNOT be further split into smaller portions. • 2. There is a void, which is empty space between atoms. • 3. Atoms are the building blocks of life.

Democritus • 4. Atoms are completely solid. • 5. Atoms are homogeneous, with no internal structure. • 6. Atoms are different in their sizes, their shapes, and their weights.


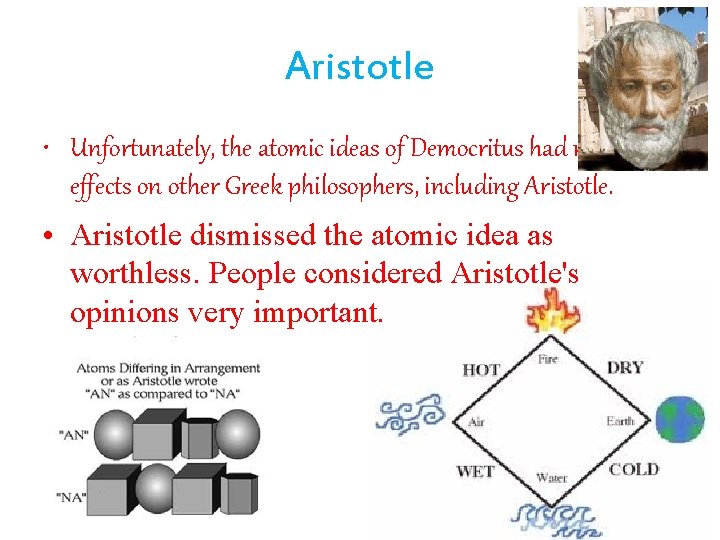
Aristotle • Unfortunately, the atomic ideas of Democritus had no lasting effects on other Greek philosophers, including Aristotle. • Aristotle dismissed the atomic idea as worthless. People considered Aristotle's opinions very important.

• Aristotle said that everything was made from four basic elements: • Earth • Air • Fire • Water He believed all substances were made of small amounts of these four elements of matter

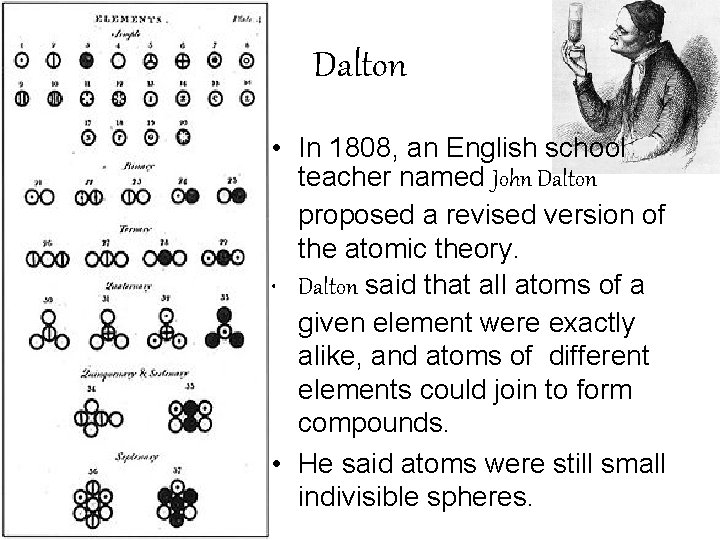
Dalton • In 1808, an English school teacher named John Dalton proposed a revised version of the atomic theory. • Dalton said that all atoms of a given element were exactly alike, and atoms of different elements could join to form compounds. • He said atoms were still small indivisible spheres.


• Unlike Democritus, Dalton based his theory on experimental evidence. Dalton • Therefore, Dalton’s theory is considered to be the foundation for modern atomic theory.

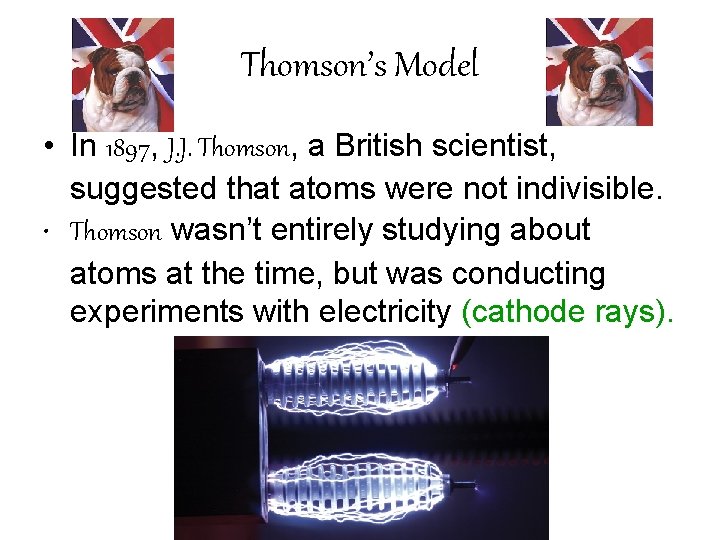
Thomson’s Model • In 1897, J. J. Thomson, a British scientist, suggested that atoms were not indivisible. • Thomson wasn’t entirely studying about atoms at the time, but was conducting experiments with electricity (cathode rays).


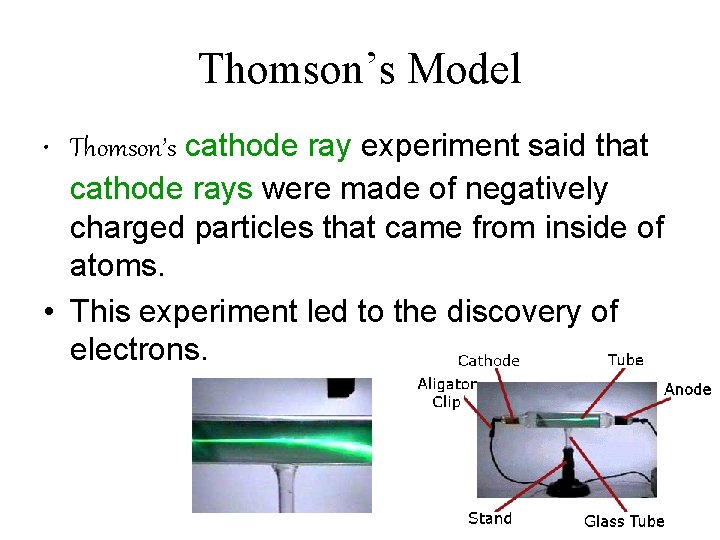
Thomson’s Model • Thomson’s cathode ray experiment said that cathode rays were made of negatively charged particles that came from inside of atoms. • This experiment led to the discovery of electrons.

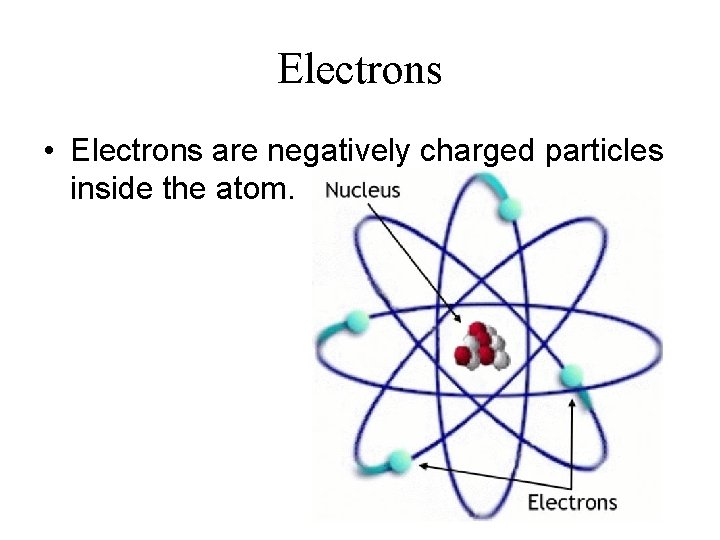
Electrons • Electrons are negatively charged particles inside the atom.

Rutherford’s Model • Ernest Rutherford, another British scientist, developed an experiment to test Thomson’s findings and stated that it needed to be revised.

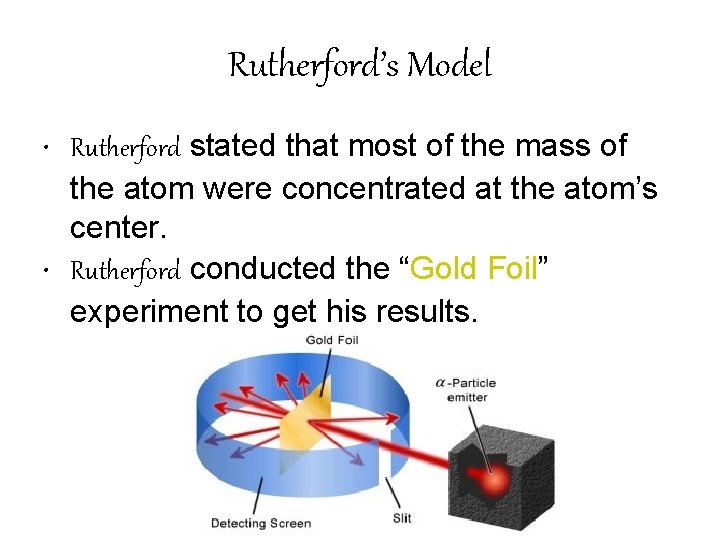
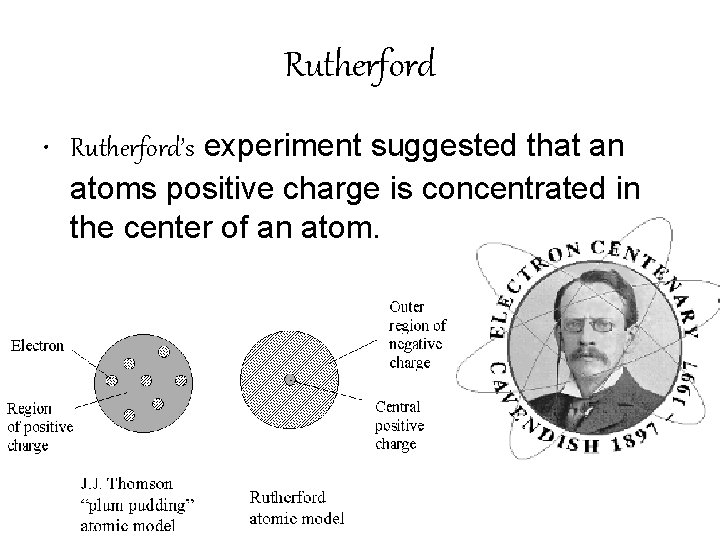
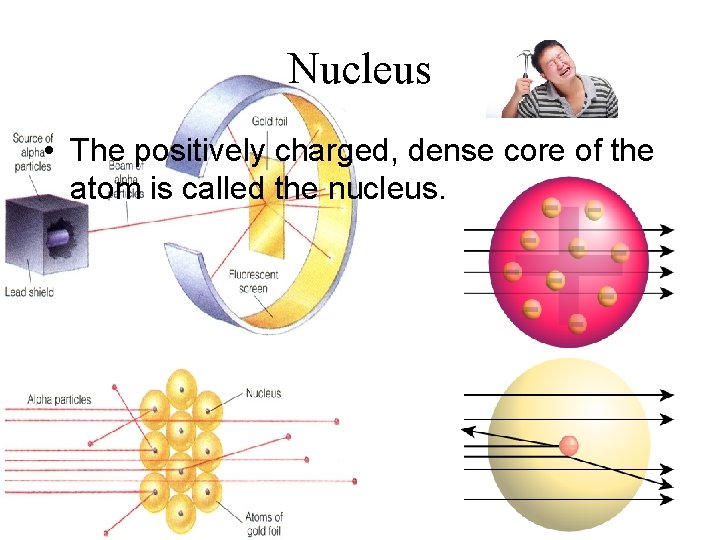
Rutherford’s Model • Rutherford stated that most of the mass of the atom were concentrated at the atom’s center. • Rutherford conducted the “Gold Foil” experiment to get his results.

Rutherford • Rutherford’s experiment suggested that an atoms positive charge is concentrated in the center of an atom.

Nucleus • The positively charged, dense core of the atom is called the nucleus.

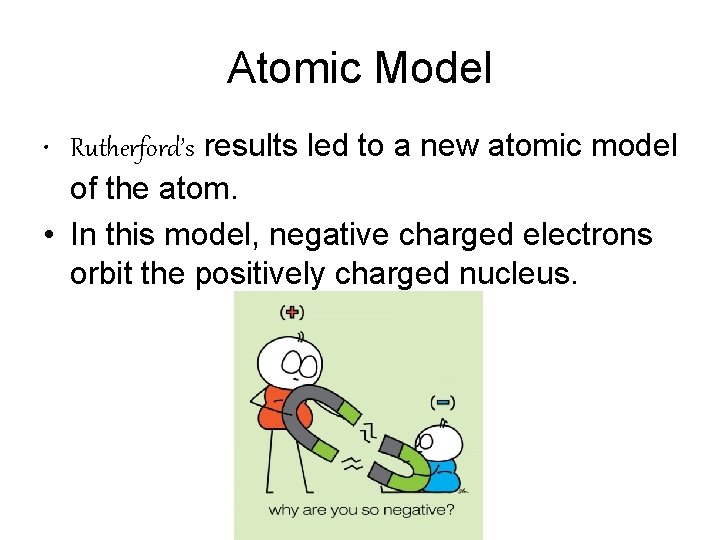
Atomic Model • Rutherford’s results led to a new atomic model of the atom. • In this model, negative charged electrons orbit the positively charged nucleus.

The END