Chapter 4 The Chemistry of Carbon AP Biology
Chapter 4. The Chemistry of Carbon AP Biology 2003 -2004
Why study Carbon? § All living things are made of cells § Cells ~72% H 2 O u ~3% salts (Na, Cl, K…) u ~25% carbon compounds w carbohydrates w lipids w proteins w nucleic acids u AP Biology 2003 -2004
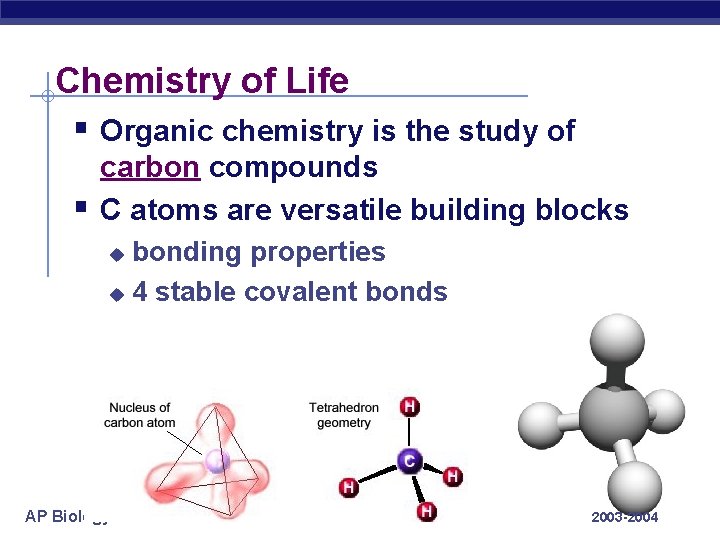
Chemistry of Life § Organic chemistry is the study of § carbon compounds C atoms are versatile building blocks bonding properties u 4 stable covalent bonds u AP Biology 2003 -2004
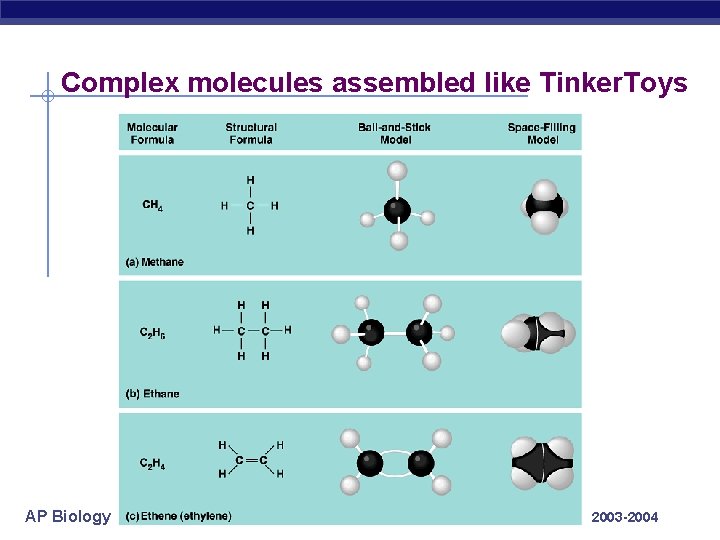
Complex molecules assembled like Tinker. Toys AP Biology 2003 -2004
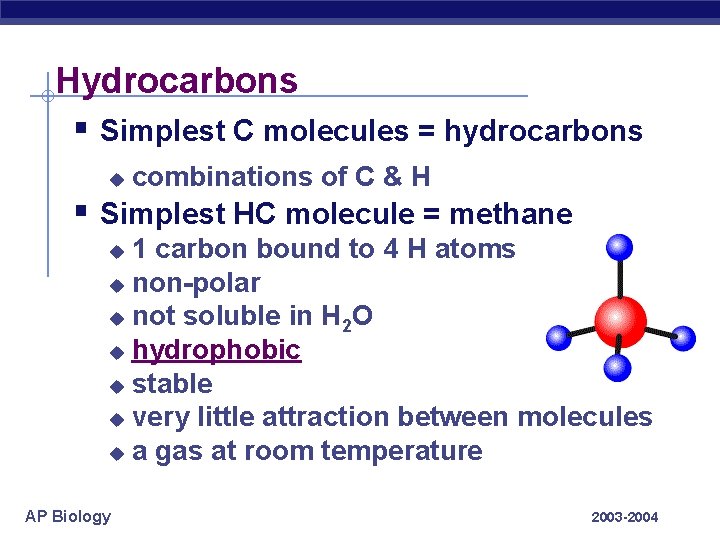
Hydrocarbons § Simplest C molecules = hydrocarbons u combinations of C & H § Simplest HC molecule = methane 1 carbon bound to 4 H atoms u non-polar u not soluble in H 2 O u hydrophobic u stable u very little attraction between molecules u a gas at room temperature u AP Biology 2003 -2004
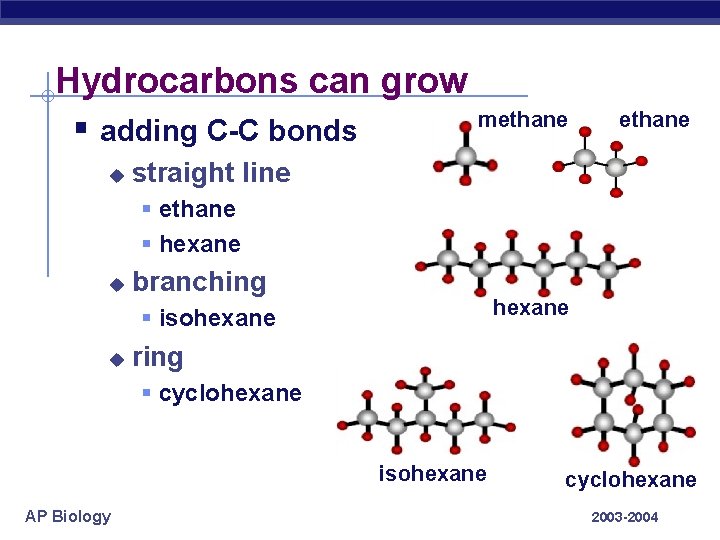
Hydrocarbons can grow § adding C-C bonds u methane straight line § ethane § hexane u branching hexane § isohexane u ring § cyclohexane isohexane AP Biology cyclohexane 2003 -2004
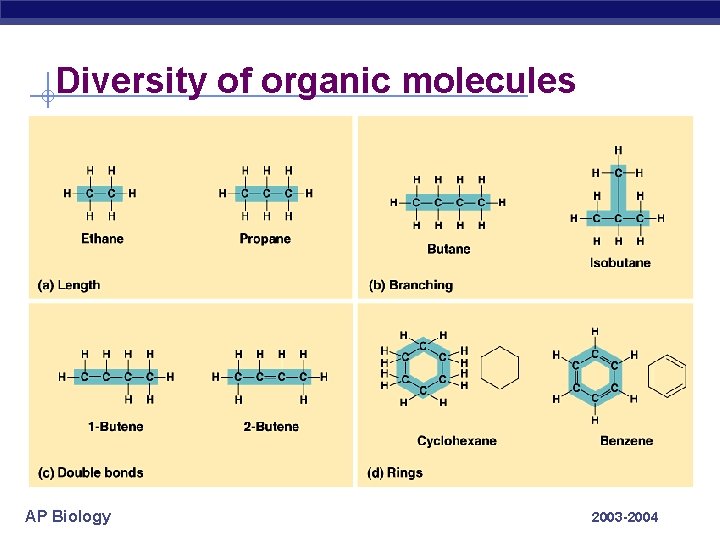
Diversity of organic molecules AP Biology 2003 -2004
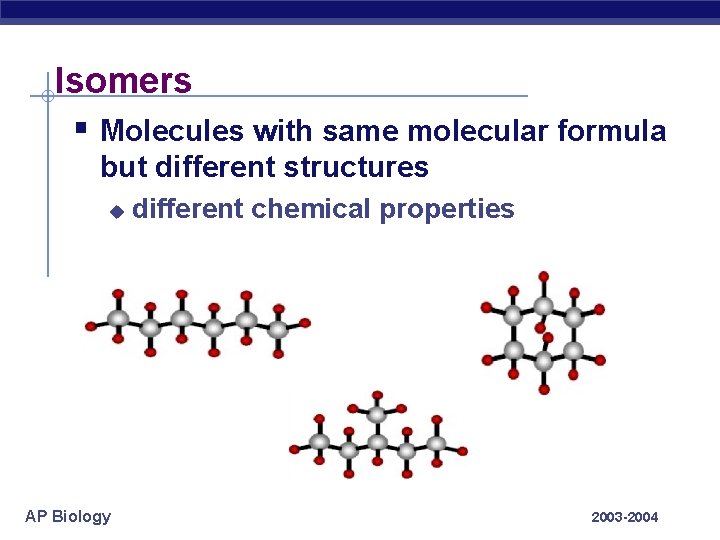
Isomers § Molecules with same molecular formula but different structures u AP Biology different chemical properties 2003 -2004
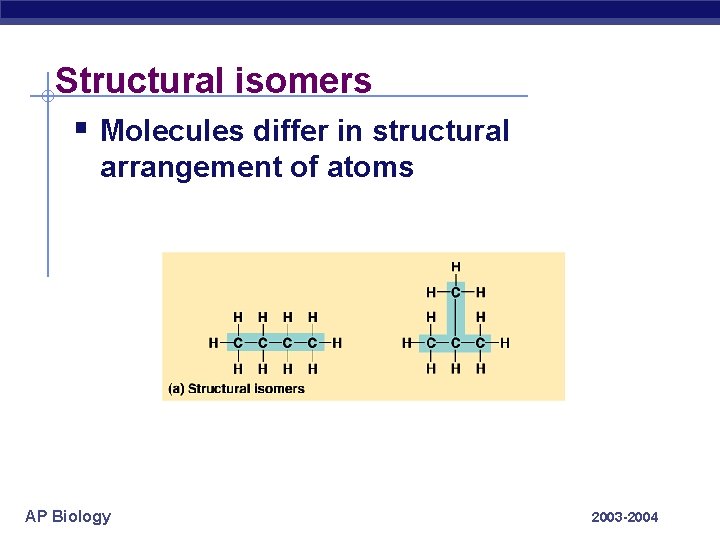
Structural isomers § Molecules differ in structural arrangement of atoms AP Biology 2003 -2004
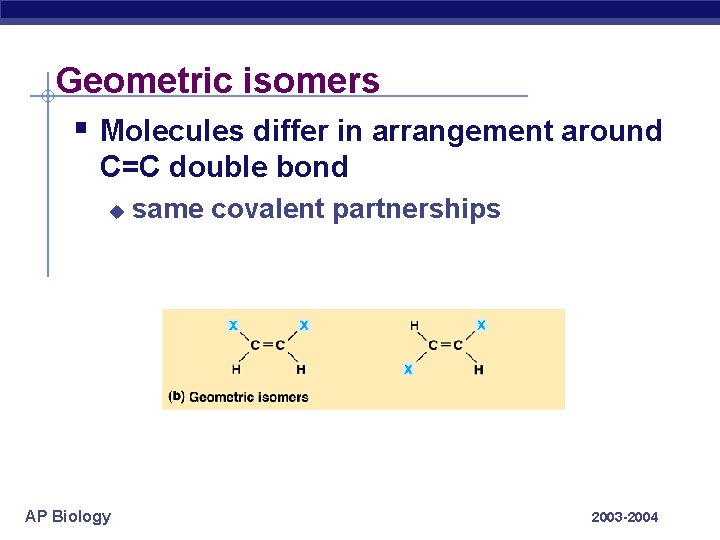
Geometric isomers § Molecules differ in arrangement around C=C double bond u AP Biology same covalent partnerships 2003 -2004
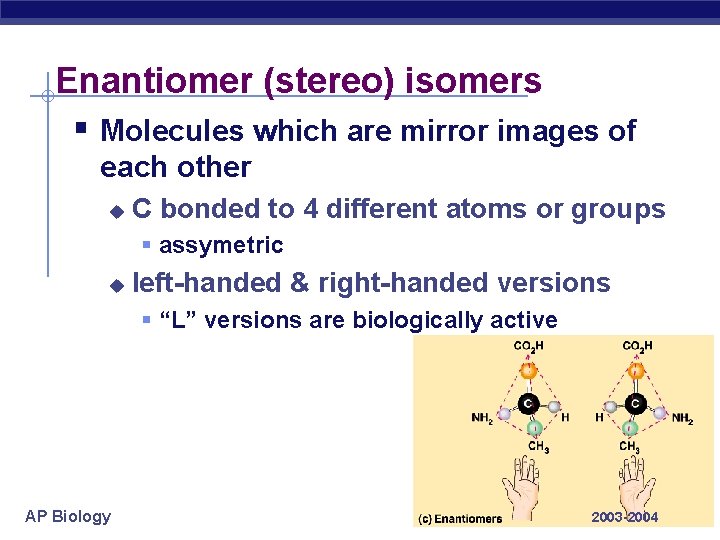
Enantiomer (stereo) isomers § Molecules which are mirror images of each other u C bonded to 4 different atoms or groups § assymetric u left-handed & right-handed versions § “L” versions are biologically active AP Biology 2003 -2004
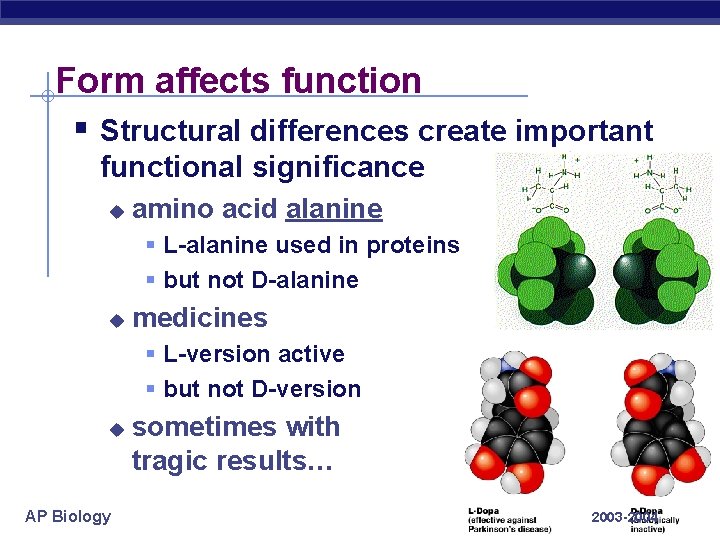
Form affects function § Structural differences create important functional significance u amino acid alanine § L-alanine used in proteins § but not D-alanine u medicines § L-version active § but not D-version u AP Biology sometimes with tragic results… 2003 -2004
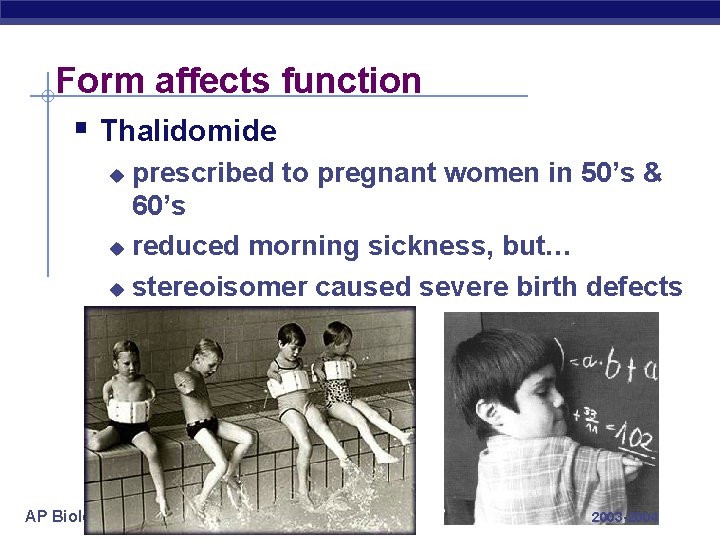
Form affects function § Thalidomide prescribed to pregnant women in 50’s & 60’s u reduced morning sickness, but… u stereoisomer caused severe birth defects u AP Biology 2003 -2004
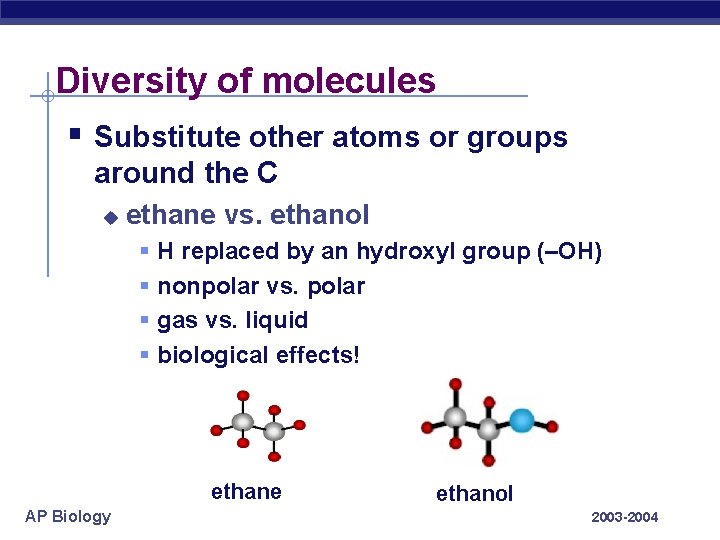
Diversity of molecules § Substitute other atoms or groups around the C u ethane vs. ethanol § § H replaced by an hydroxyl group (–OH) nonpolar vs. polar gas vs. liquid biological effects! ethane AP Biology ethanol 2003 -2004
Functional Groups AP Biology 2003 -2004
Functional groups § Components of organic molecules that are involved in chemical reactions give organic molecules distinctive properties u ex: male & female hormones… u AP Biology 2003 -2004
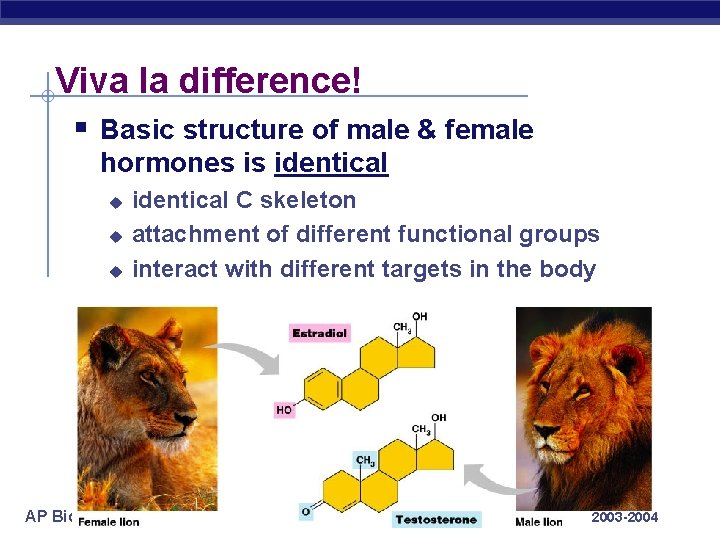
Viva la difference! § Basic structure of male & female hormones is identical u u u AP Biology identical C skeleton attachment of different functional groups interact with different targets in the body 2003 -2004
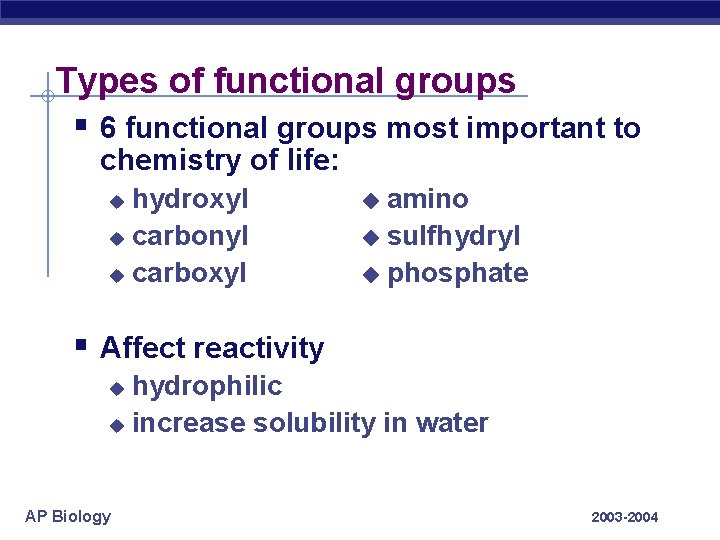
Types of functional groups § 6 functional groups most important to chemistry of life: hydroxyl u carbonyl u carboxyl u amino u sulfhydryl u phosphate u § Affect reactivity hydrophilic u increase solubility in water u AP Biology 2003 -2004
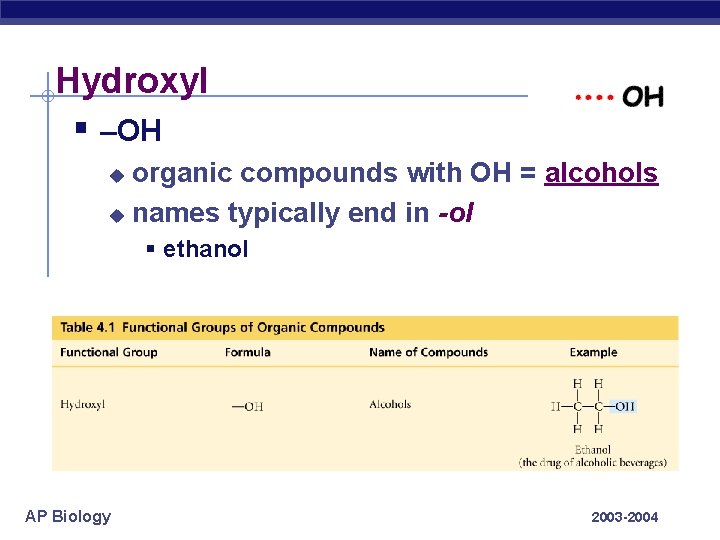
Hydroxyl § –OH organic compounds with OH = alcohols u names typically end in -ol u § ethanol AP Biology 2003 -2004
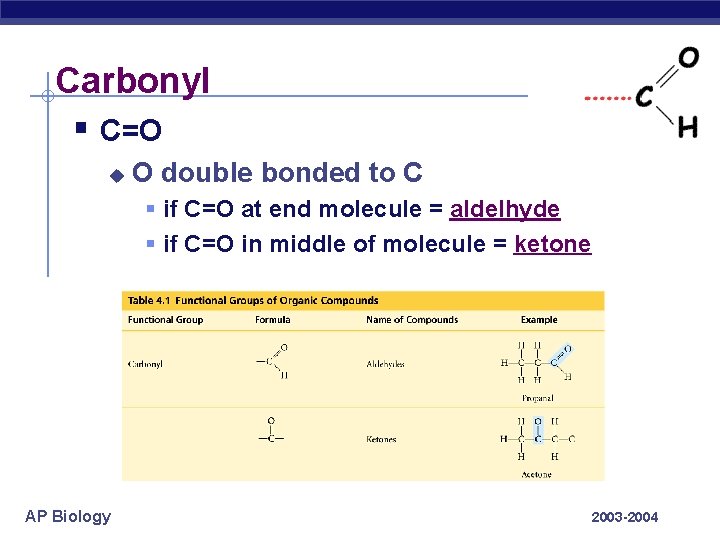
Carbonyl § C=O u O double bonded to C § if C=O at end molecule = aldelhyde § if C=O in middle of molecule = ketone AP Biology 2003 -2004
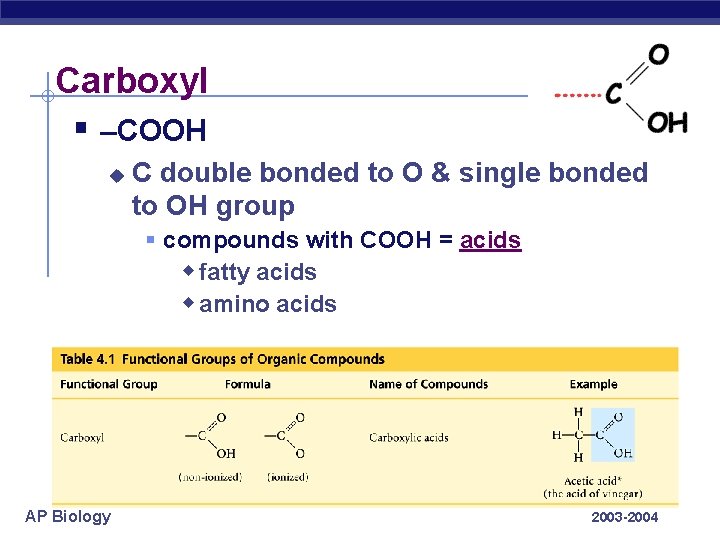
Carboxyl § –COOH u C double bonded to O & single bonded to OH group § compounds with COOH = acids w fatty acids w amino acids AP Biology 2003 -2004
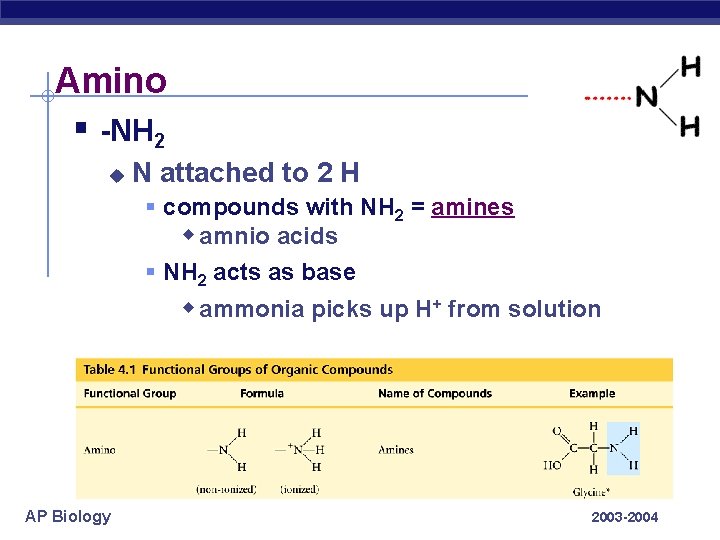
Amino § -NH 2 u N attached to 2 H § compounds with NH 2 = amines w amnio acids § NH 2 acts as base w ammonia picks up H+ from solution AP Biology 2003 -2004
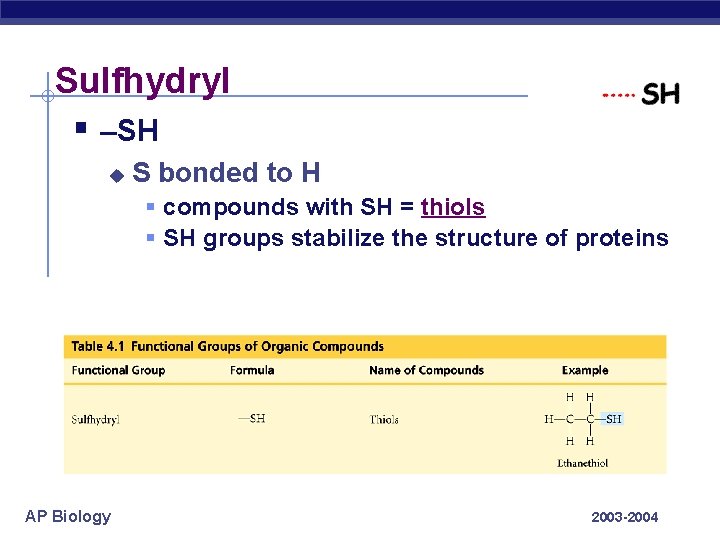
Sulfhydryl § –SH u S bonded to H § compounds with SH = thiols § SH groups stabilize the structure of proteins AP Biology 2003 -2004
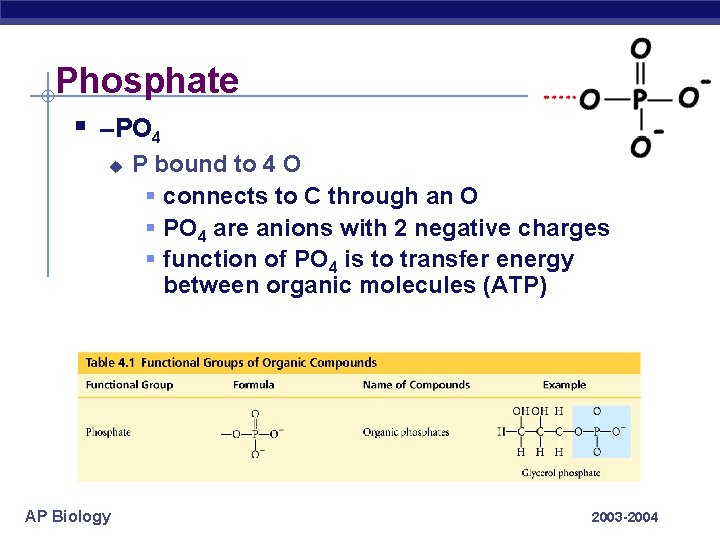
Phosphate § –PO 4 u AP Biology P bound to 4 O § connects to C through an O § PO 4 are anions with 2 negative charges § function of PO 4 is to transfer energy between organic molecules (ATP) 2003 -2004

Why study Functional Groups? § These are the building blocks for biological molecules …and that comes next! AP Biology 2003 -2004

Any Questions? ? AP Biology 2003 -2004
- Slides: 26