Chapter 4 semester 12014 Reactions in Aqueous Solution


- Slides: 24

Chapter 4 (semester 1/2014) Reactions in Aqueous Solution prepared by A. Kyi Tin Ref: Raymong Chang. Chemistry Eleventh Edition, Mc. Graw – Hill International Edition 4. 1 General Properties of Aqueous Solutions 4. 2 Precipitation Reactions 4. 3 Acid- Base Reactions 4. 4 Oxidation – Reduction Reactions 4. 5 Concentration of Solutions

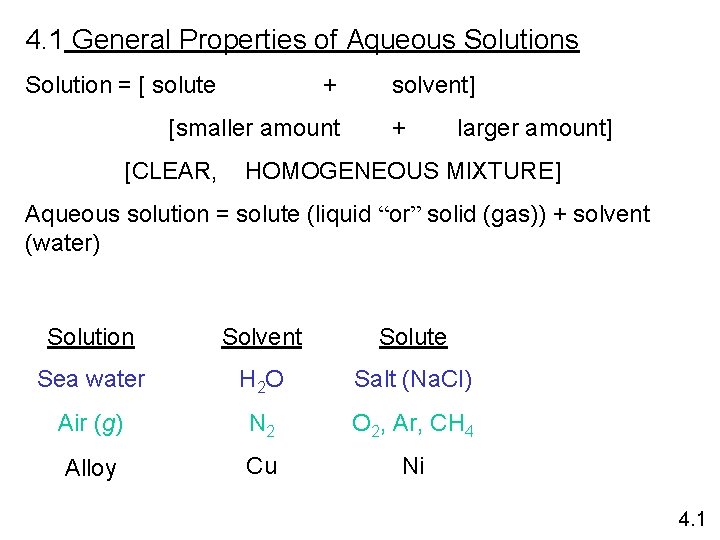
4. 1 General Properties of Aqueous Solution = [ solute + [smaller amount [CLEAR, solvent] + larger amount] HOMOGENEOUS MIXTURE] Aqueous solution = solute (liquid “or” solid (gas)) + solvent (water) Solution Solvent Solute Sea water H 2 O Salt (Na. Cl) Air (g) N 2 O 2, Ar, CH 4 Alloy Cu Ni 4. 1

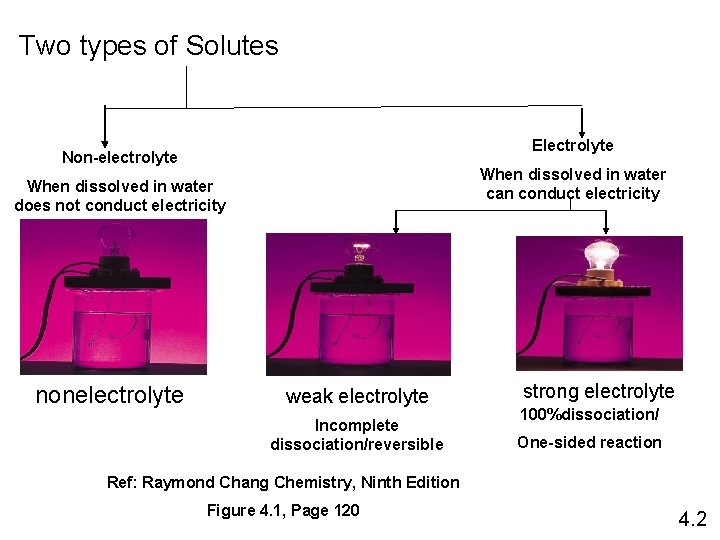
Two types of Solutes Electrolyte Non-electrolyte When dissolved in water can conduct electricity When dissolved in water does not conduct electricity nonelectrolyte weak electrolyte Incomplete dissociation/reversible strong electrolyte 100%dissociation/ One-sided reaction Ref: Raymond Chang Chemistry, Ninth Edition Figure 4. 1, Page 120 4. 2

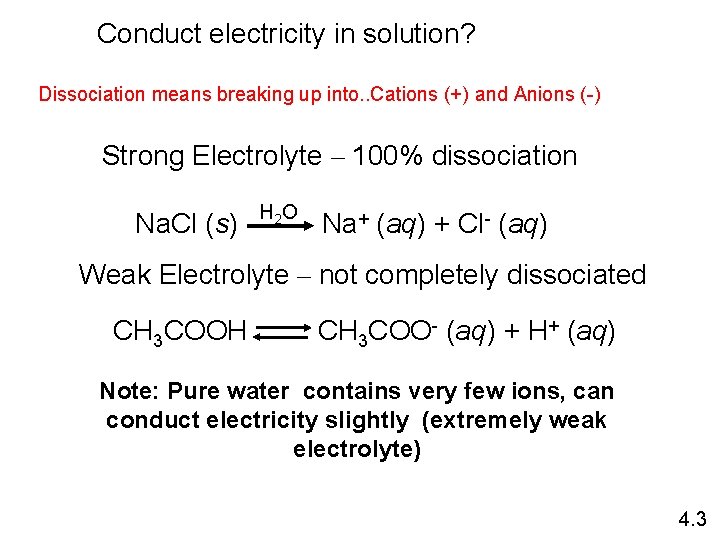
Conduct electricity in solution? Dissociation means breaking up into. . Cations (+) and Anions (-) Strong Electrolyte – 100% dissociation Na. Cl (s) H 2 O Na+ (aq) + Cl- (aq) Weak Electrolyte – not completely dissociated CH 3 COOH CH 3 COO- (aq) + H+ (aq) Note: Pure water contains very few ions, can conduct electricity slightly (extremely weak electrolyte) 4. 3

Hydration: is the process in which an ion is surrounded by water molecules arranged in a specific manner. Water, electrically neutral molecule has a positive poles and negative poles, it is a polar solvent. Ex: when Na. Cl dissolves in water Na+ ions and Clions are separated from each other and undergo “hydration”. Hydration helps to stabilize ions in solution and prevents cations from combining with anions. 4. 4

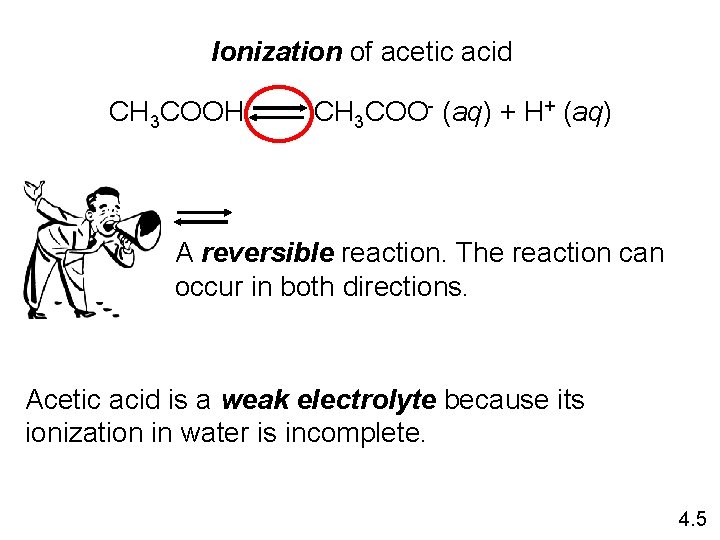
Ionization of acetic acid CH 3 COOH CH 3 COO- (aq) + H+ (aq) A reversible reaction. The reaction can occur in both directions. Acetic acid is a weak electrolyte because its ionization in water is incomplete. 4. 5

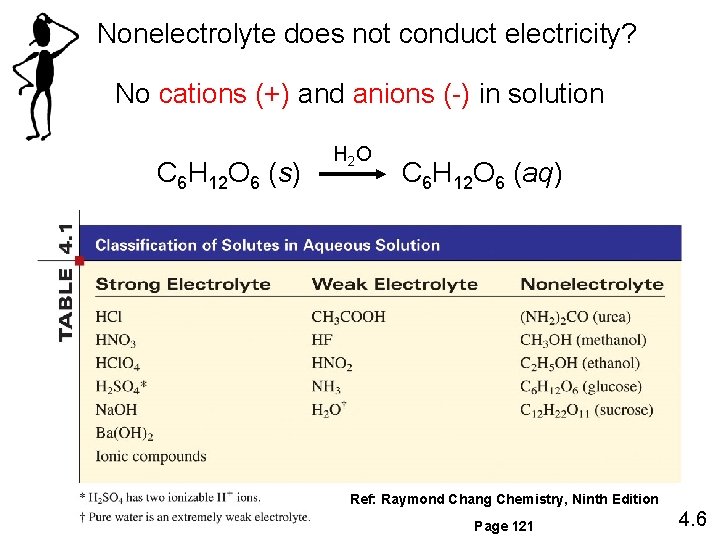
Nonelectrolyte does not conduct electricity? No cations (+) and anions (-) in solution C 6 H 12 O 6 (s) H 2 O C 6 H 12 O 6 (aq) Ref: Raymond Chang Chemistry, Ninth Edition Page 121 4. 6

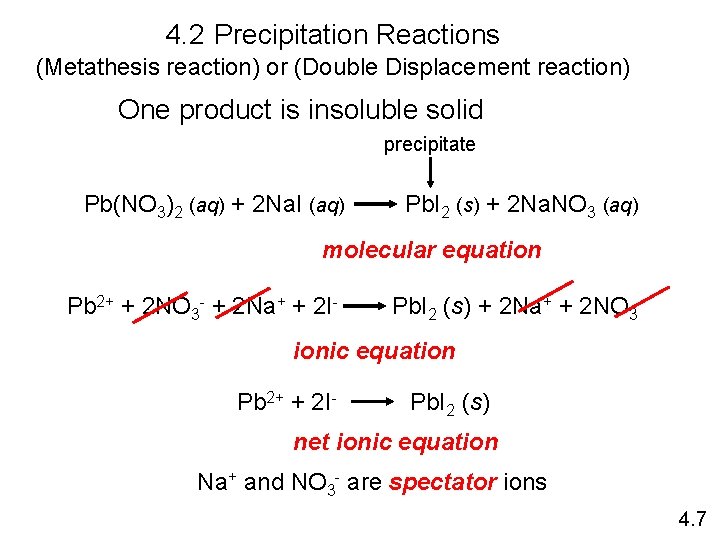
4. 2 Precipitation Reactions (Metathesis reaction) or (Double Displacement reaction) One product is insoluble solid precipitate Pb(NO 3)2 (aq) + 2 Na. I (aq) Pb. I 2 (s) + 2 Na. NO 3 (aq) molecular equation Pb 2+ + 2 NO 3 - + 2 Na+ + 2 I- Pb. I 2 (s) + 2 Na+ + 2 NO 3 - ionic equation Pb 2+ + 2 I- Pb. I 2 (s) net ionic equation Na+ and NO 3 - are spectator ions 4. 7

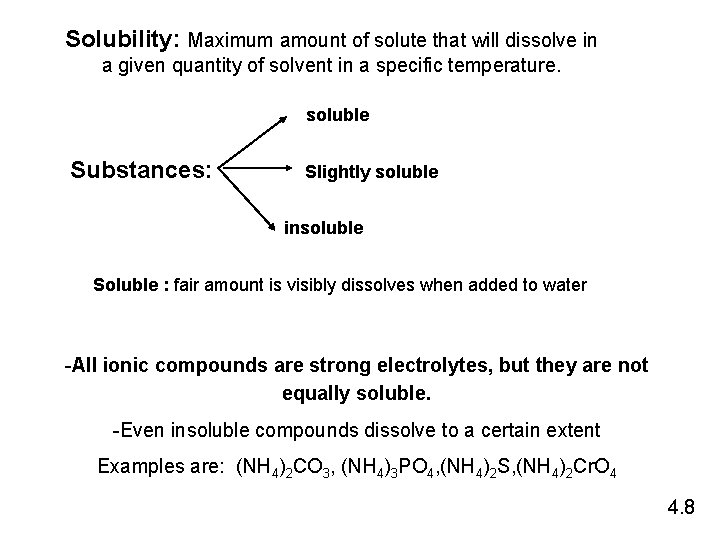
Solubility: Maximum amount of solute that will dissolve in a given quantity of solvent in a specific temperature. soluble Substances: Slightly soluble insoluble Soluble : fair amount is visibly dissolves when added to water -All ionic compounds are strong electrolytes, but they are not equally soluble. -Even insoluble compounds dissolve to a certain extent Examples are: (NH 4)2 CO 3, (NH 4)3 PO 4, (NH 4)2 S, (NH 4)2 Cr. O 4 4. 8

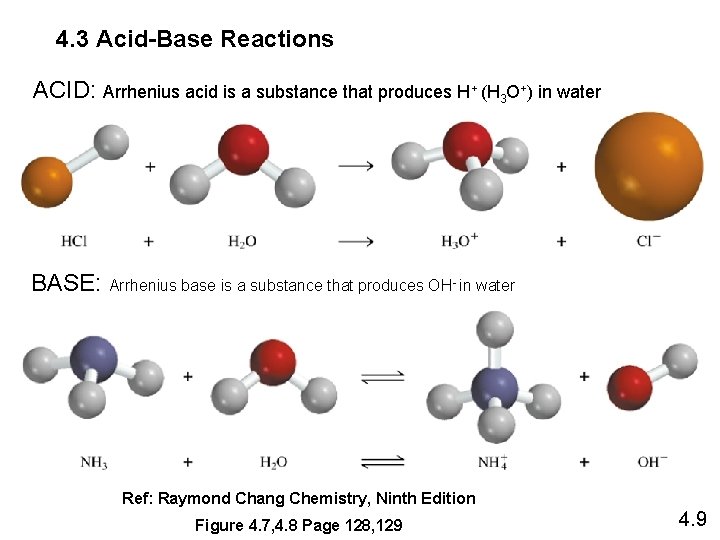
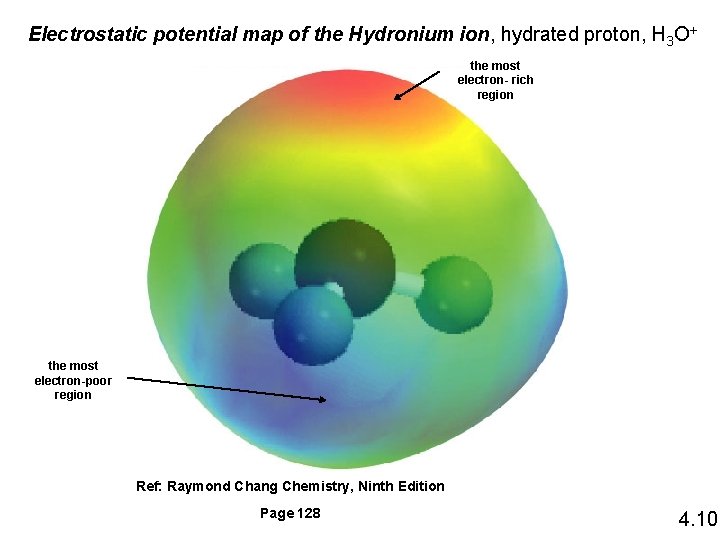
4. 3 Acid-Base Reactions ACID: Arrhenius acid is a substance that produces H+ (H 3 O+) in water BASE: Arrhenius base is a substance that produces OH- in water Ref: Raymond Chang Chemistry, Ninth Edition Figure 4. 7, 4. 8 Page 128, 129 4. 9

Electrostatic potential map of the Hydronium ion, hydrated proton, H 3 O+ the most electron- rich region the most electron-poor region Ref: Raymond Chang Chemistry, Ninth Edition Page 128 4. 10

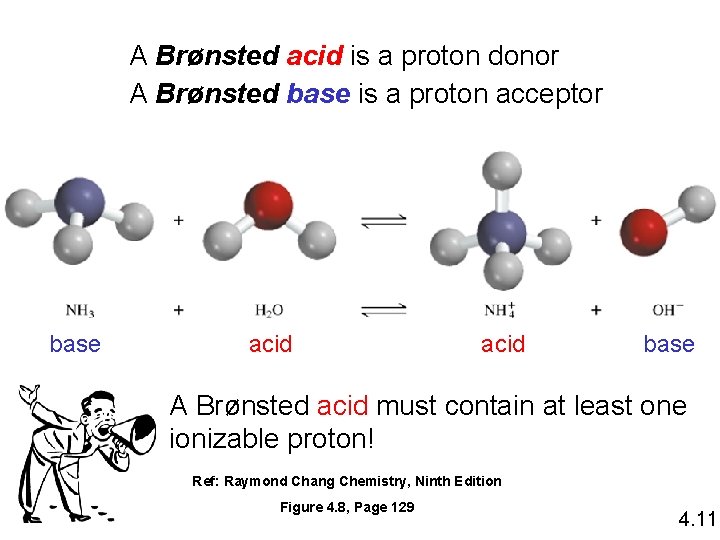
A Brønsted acid is a proton donor A Brønsted base is a proton acceptor base acid base A Brønsted acid must contain at least one ionizable proton! Ref: Raymond Chang Chemistry, Ninth Edition Figure 4. 8, Page 129 4. 11

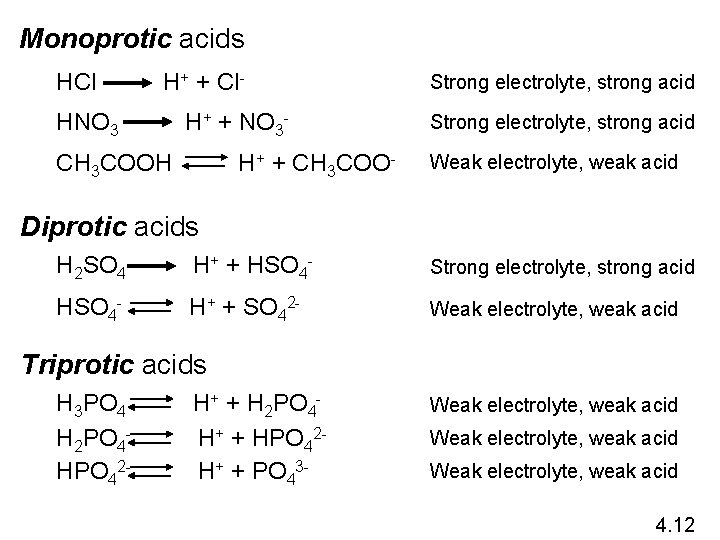
Monoprotic acids HCl H+ + Cl- HNO 3 H+ + NO 3 - CH 3 COOH H+ + CH 3 COO- Strong electrolyte, strong acid Weak electrolyte, weak acid Diprotic acids H 2 SO 4 H+ + HSO 4 - Strong electrolyte, strong acid HSO 4 - H+ + SO 42 - Weak electrolyte, weak acid Triprotic acids H 3 PO 4 H 2 PO 4 HPO 42 - H+ + H 2 PO 4 H+ + HPO 42 H+ + PO 43 - Weak electrolyte, weak acid 4. 12

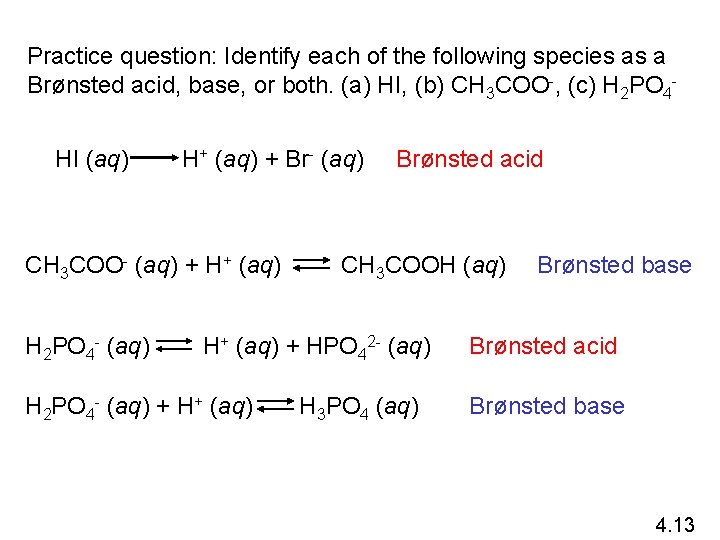
Practice question: Identify each of the following species as a Brønsted acid, base, or both. (a) HI, (b) CH 3 COO-, (c) H 2 PO 4 HI (aq) H+ (aq) + Br- (aq) CH 3 COO- (aq) + H+ (aq) H 2 PO 4 - (aq) Brønsted acid CH 3 COOH (aq) H+ (aq) + HPO 42 - (aq) H 2 PO 4 - (aq) + H+ (aq) H 3 PO 4 (aq) Brønsted base Brønsted acid Brønsted base 4. 13

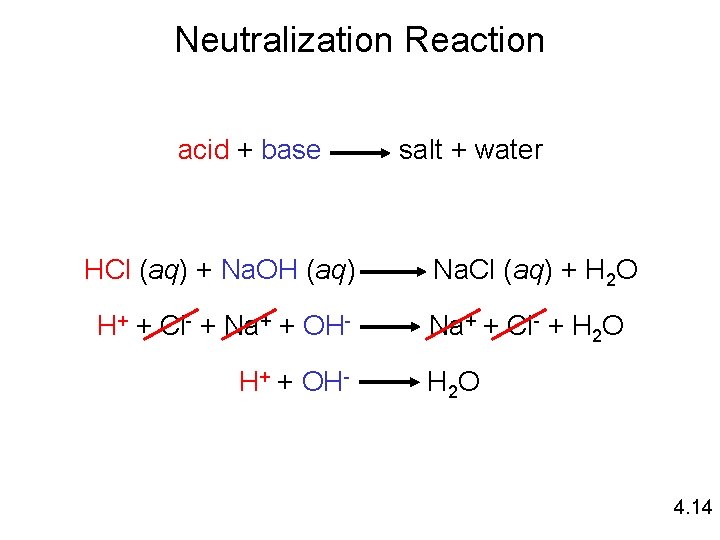
Neutralization Reaction acid + base HCl (aq) + Na. OH (aq) H+ + Cl- + Na+ + OHH+ + OH- salt + water Na. Cl (aq) + H 2 O Na+ + Cl- + H 2 O 4. 14

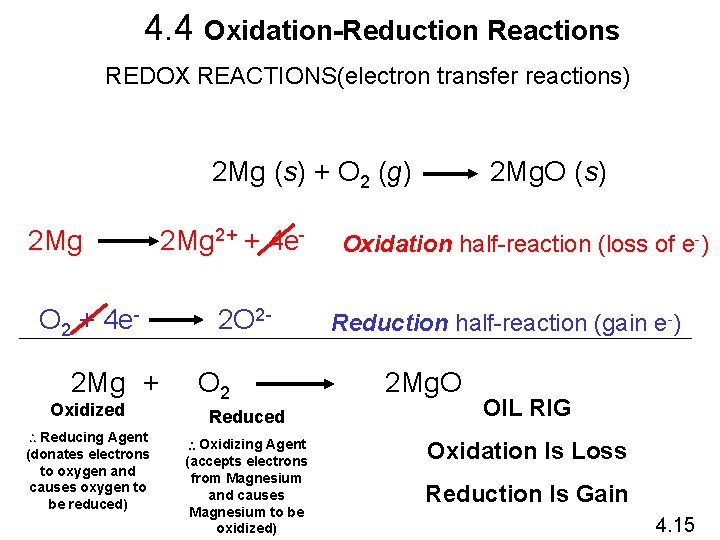
4. 4 Oxidation-Reduction Reactions REDOX REACTIONS(electron transfer reactions) 2 Mg (s) + O 2 (g) 2 Mg O 2 + 4 e 2 Mg + Oxidized Reducing Agent (donates electrons to oxygen and causes oxygen to be reduced) 2 Mg 2+ + 4 e 2 O 2 O 2 Reduced Oxidizing Agent (accepts electrons from Magnesium and causes Magnesium to be oxidized) 2 Mg. O (s) Oxidation half-reaction (loss of e-) Reduction half-reaction (gain e-) 2 Mg. O OIL RIG Oxidation Is Loss Reduction Is Gain 4. 15

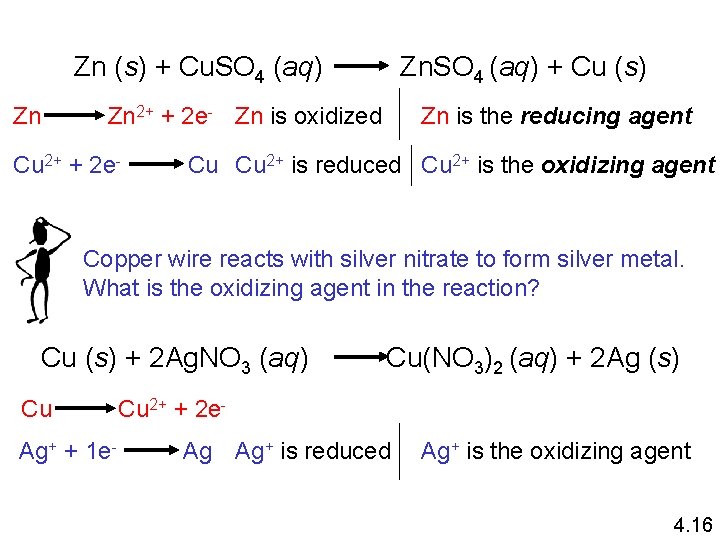
Zn (s) + Cu. SO 4 (aq) Zn Zn. SO 4 (aq) + Cu (s) Zn is the reducing agent Zn 2+ + 2 e- Zn is oxidized Cu 2+ + 2 e- Cu Cu 2+ is reduced Cu 2+ is the oxidizing agent Copper wire reacts with silver nitrate to form silver metal. What is the oxidizing agent in the reaction? Cu (s) + 2 Ag. NO 3 (aq) Cu Ag+ + 1 e- Cu(NO 3)2 (aq) + 2 Ag (s) Cu 2+ + 2 e. Ag Ag+ is reduced Ag+ is the oxidizing agent 4. 16

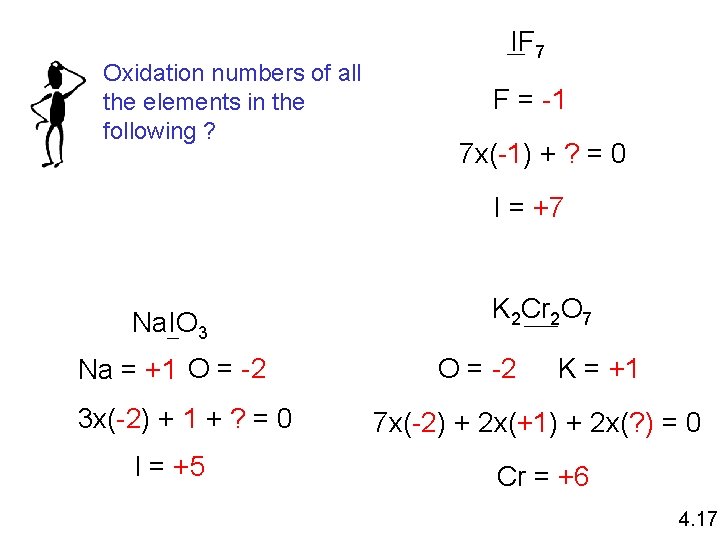
Oxidation numbers of all the elements in the following ? IF 7 F = -1 7 x(-1) + ? = 0 I = +7 Na. IO 3 Na = +1 O = -2 3 x(-2) + 1 + ? = 0 I = +5 K 2 Cr 2 O 7 O = -2 K = +1 7 x(-2) + 2 x(+1) + 2 x(? ) = 0 Cr = +6 4. 17

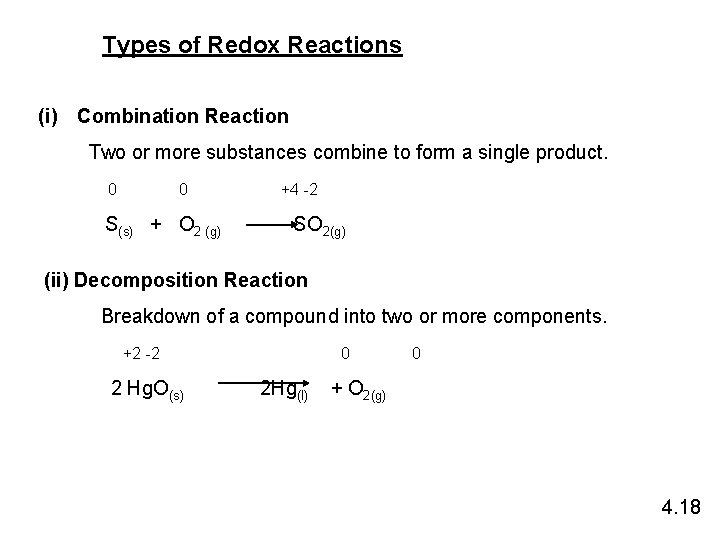
Types of Redox Reactions (i) Combination Reaction Two or more substances combine to form a single product. 0 0 S(s) + O 2 (g) +4 -2 SO 2(g) (ii) Decomposition Reaction Breakdown of a compound into two or more components. +2 -2 2 Hg. O(s) 0 2 Hg(l) 0 + O 2(g) 4. 18

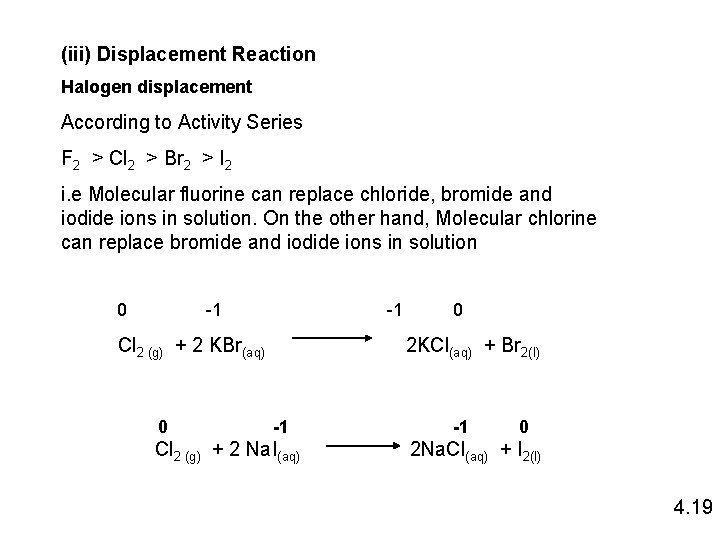
(iii) Displacement Reaction Halogen displacement According to Activity Series F 2 > Cl 2 > Br 2 > I 2 i. e Molecular fluorine can replace chloride, bromide and iodide ions in solution. On the other hand, Molecular chlorine can replace bromide and iodide ions in solution 0 -1 -1 Cl 2 (g) + 2 KBr(aq) 0 0 2 KCl(aq) + Br 2(l) -1 Cl 2 (g) + 2 Na. I(aq) -1 0 2 Na. Cl(aq) + I 2(l) 4. 19

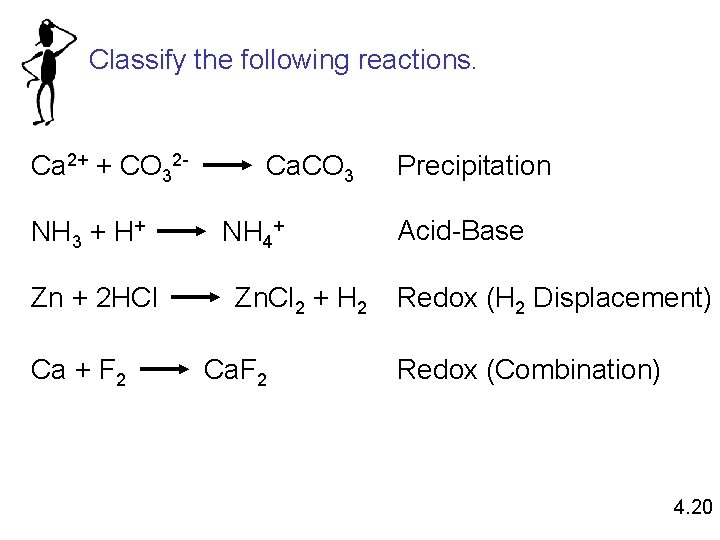
Classify the following reactions. Ca 2+ + CO 32 NH 3 + H+ Zn + 2 HCl Ca + F 2 Ca. CO 3 NH 4+ Zn. Cl 2 + H 2 Ca. F 2 Precipitation Acid-Base Redox (H 2 Displacement) Redox (Combination) 4. 20

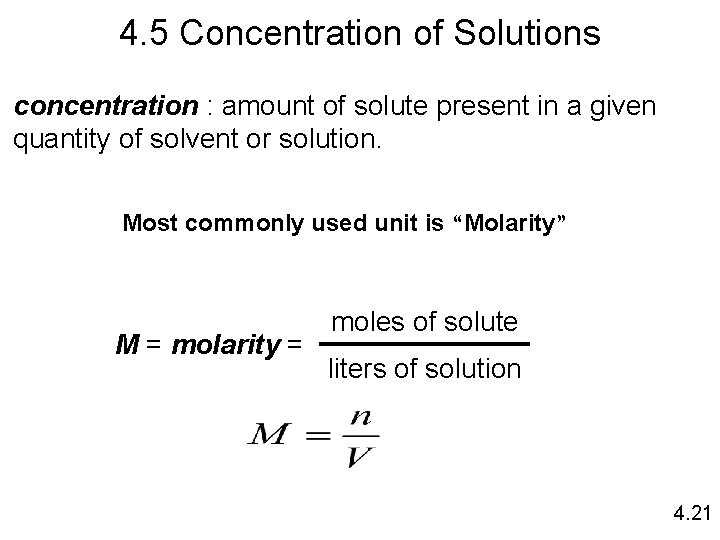
4. 5 Concentration of Solutions concentration : amount of solute present in a given quantity of solvent or solution. Most commonly used unit is “Molarity” M = molarity = moles of solute liters of solution 4. 21

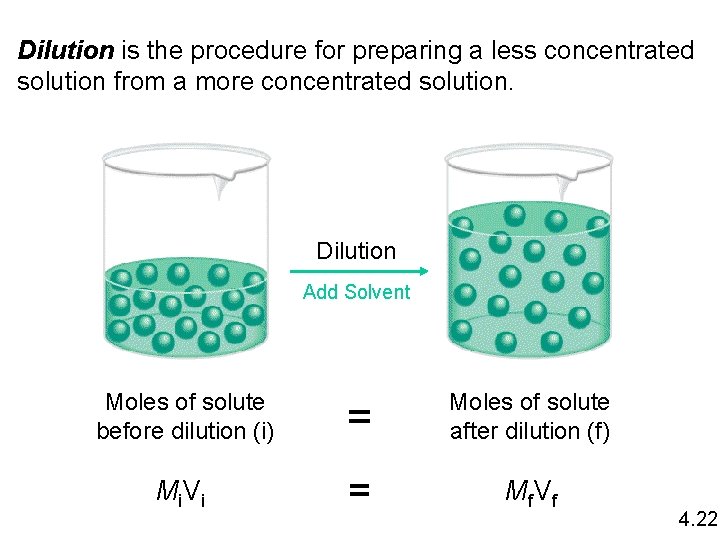
Dilution is the procedure for preparing a less concentrated solution from a more concentrated solution. Dilution Add Solvent Moles of solute before dilution (i) = Moles of solute after dilution (f) Mi V i = Mf V f 4. 22

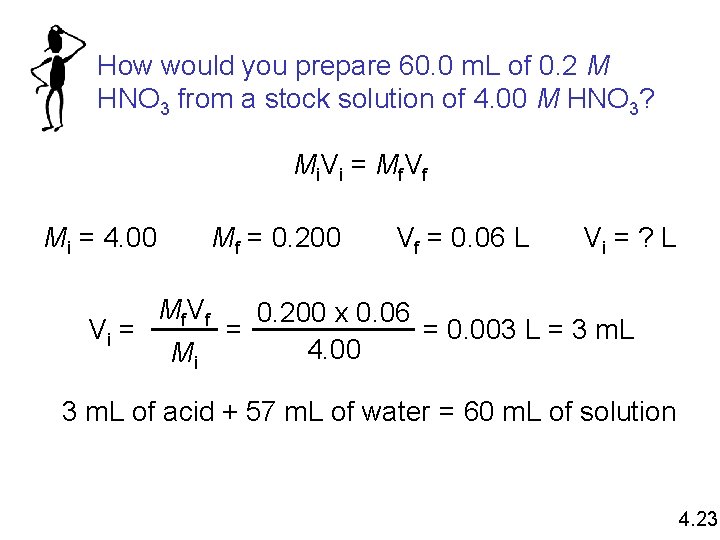
How would you prepare 60. 0 m. L of 0. 2 M HNO 3 from a stock solution of 4. 00 M HNO 3? Mi V i = Mf V f Mi = 4. 00 Vi = Mf = 0. 200 Mf V f Mi Vf = 0. 06 L Vi = ? L 0. 200 x 0. 06 = 0. 003 L = 3 m. L = 4. 00 3 m. L of acid + 57 m. L of water = 60 m. L of solution 4. 23