Chapter 4 Reactions in Aqueous Solutions Aqueous Solution





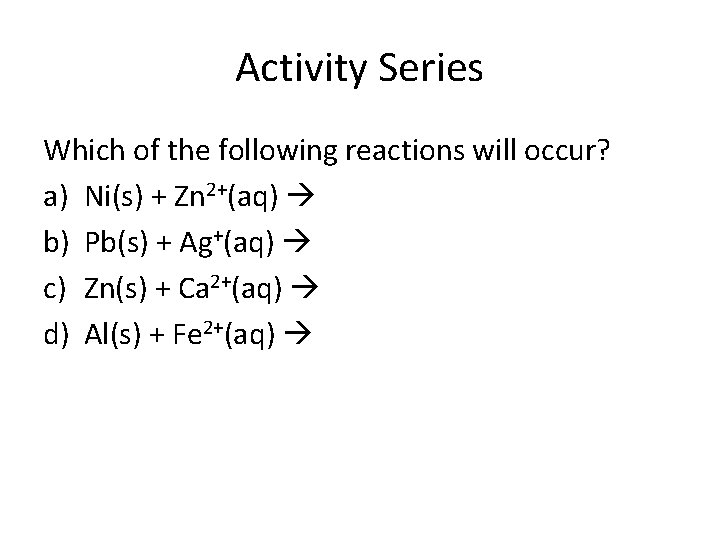




- Slides: 22

Chapter 4 – Reactions in Aqueous Solutions

Aqueous Solution – homogenous mixture of two or more substances. Solute - substance that is being dissolved. (present in smaller amounts) Solvent – usually water ( present in larger amounts) Electrolyte – substance that can dissolve in water & can conduct electricity. Non electrolyte – substance that may dissolve in water & does not conduct electricity. 2

Electrolytes Strong Electrolytes – conduct electricity very efficiently (bulb shines brightly). 100% dissociation in water. Ex: Strong acids & bases Weak Electrolytes – conduct only a small amount of electricity (bulb glows dimly). Not completely dissociated in water. Ex: acetic acid Non electrolytes – no current flows (bulb remains unlit). Dissolves but does not produce any ions. 3

Precipitation Reactions or double displacement reactions A double displacement reaction in which a precipitate forms (insoluble substance) and separates from the solution. Soluble – solid dissolves in solution; (aq) is used in reaction equation. Insoluble – solid does not dissolve in solution; (s) is used in reaction equation.

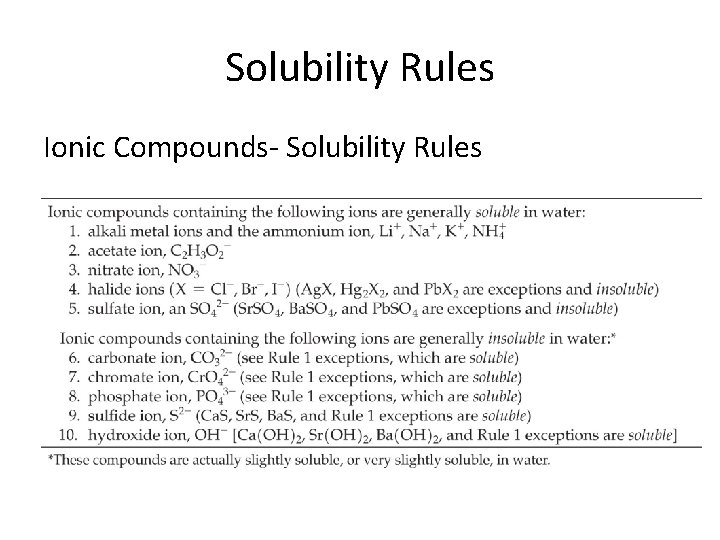
Solubility Rules Ionic Compounds- Solubility Rules

Soluble/ Insoluble 1. 2. 3. 4. 5. 6. Ni. Cl 2 Ag 2 S Cs 3 PO 4 (NH 4)2 SO 4 Pb. Cl 2 Ba(OH)2

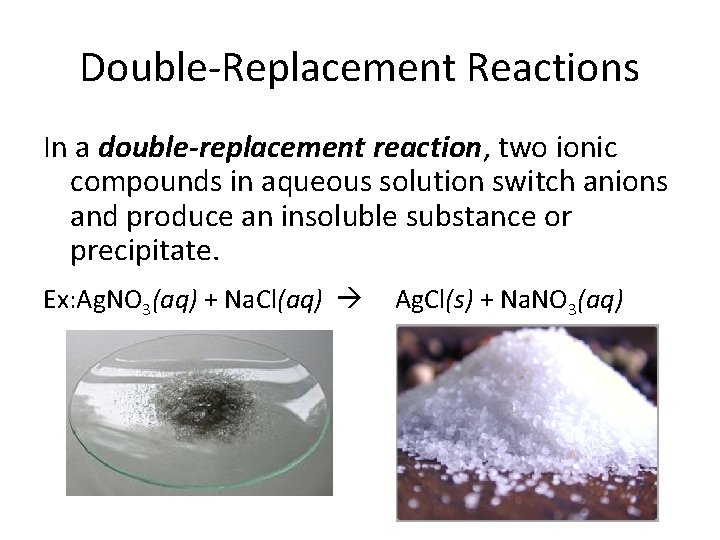
Double-Replacement Reactions In a double-replacement reaction, two ionic compounds in aqueous solution switch anions and produce an insoluble substance or precipitate. Ex: Ag. NO 3(aq) + Na. Cl(aq) Ag. Cl(s) + Na. NO 3(aq)

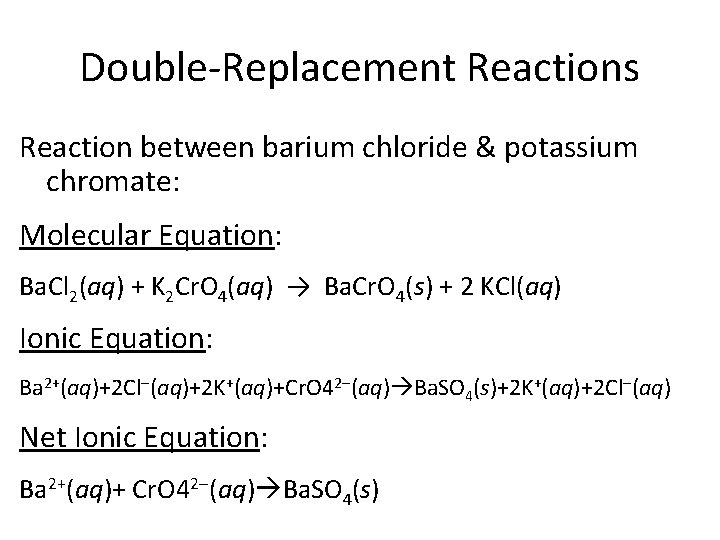
Double-Replacement Reactions Reaction between barium chloride & potassium chromate: Molecular Equation: Ba. Cl 2(aq) + K 2 Cr. O 4(aq) → Ba. Cr. O 4(s) + 2 KCl(aq) Ionic Equation: Ba 2+(aq)+2 Cl-(aq)+2 K+(aq)+Cr. O 42 -(aq) Ba. SO 4(s)+2 K+(aq)+2 Cl-(aq) Net Ionic Equation: Ba 2+(aq)+ Cr. O 42 -(aq) Ba. SO 4(s)

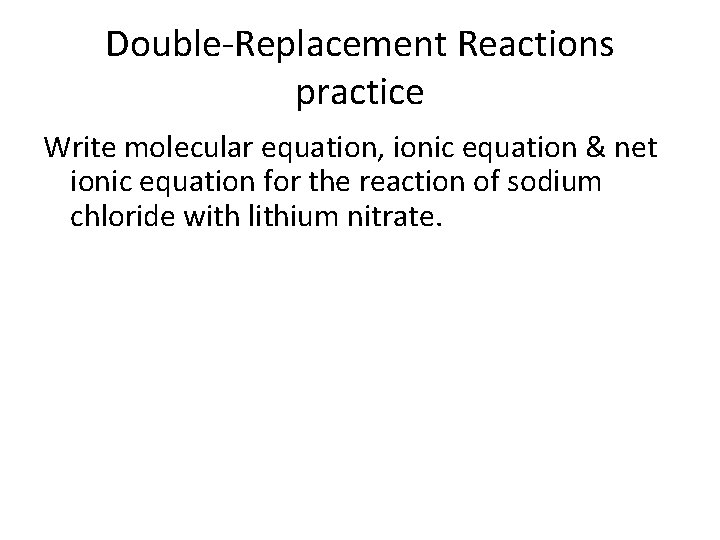
Double-Replacement Reactions practice Write molecular equation, ionic equation & net ionic equation for the reaction of sodium chloride with lithium nitrate.

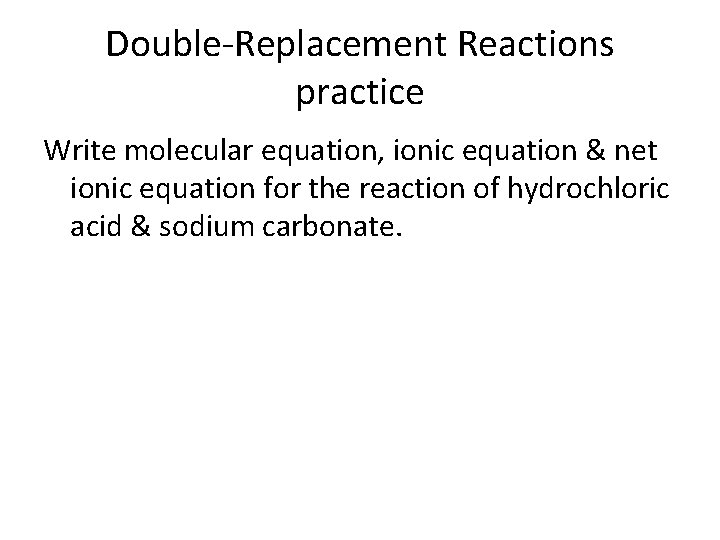
Double-Replacement Reactions practice Write molecular equation, ionic equation & net ionic equation for the reaction of hydrochloric acid & sodium carbonate.

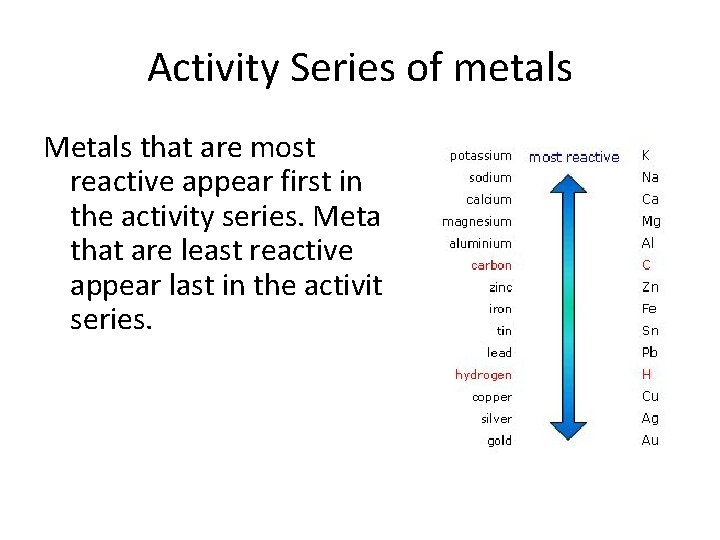
Activity Series When a metal undergoes a single replacement reaction, it displaces another metal from a compound or aqueous solution. The more active metal can displace the other metal. Activity series - metals are arranged according to its ability to undergo a reaction.

Activity Series of metals Metals that are most reactive appear first in the activity series. Metals that are least reactive appear last in the activity series.
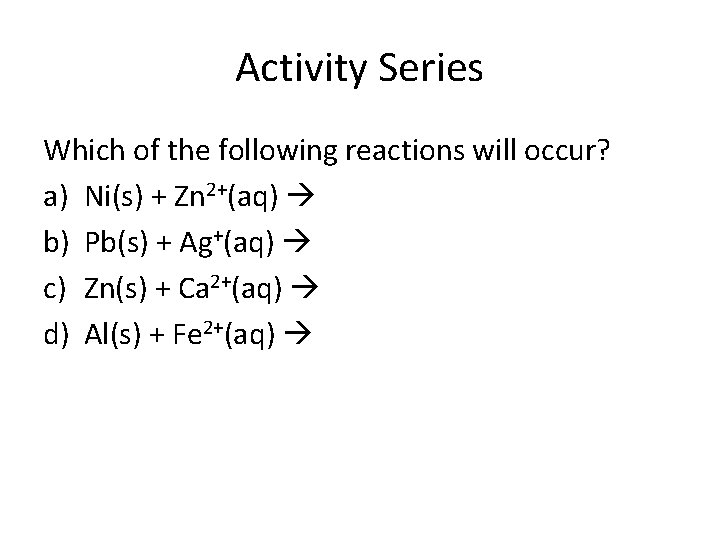
Activity Series Which of the following reactions will occur? a) Ni(s) + Zn 2+(aq) b) Pb(s) + Ag+(aq) c) Zn(s) + Ca 2+(aq) d) Al(s) + Fe 2+(aq)

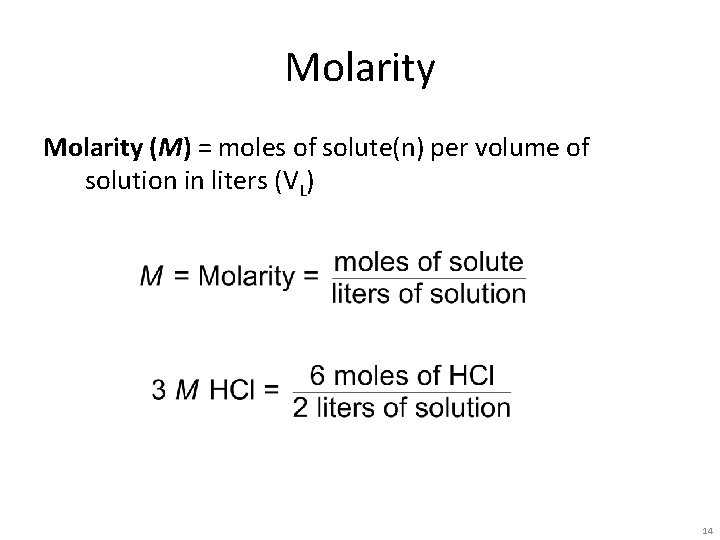
Molarity (M) = moles of solute(n) per volume of solution in liters (VL) 14

Molarity Practice How many m. L of 1. 50 M KOH solution are needed to supply 0. 125 mol of KOH?

Dilution is the procedure for preparing a less concentrated solution from a more concentrated solution. Moles of solute before dilution = moles of solute after dilution M 1 V 1 = M 2 V 2

Dilution Practice If 200 m. L of a 2. 5 M Na. OH solution is diluted to 500 m. L, what is the new concentration of Na. OH?

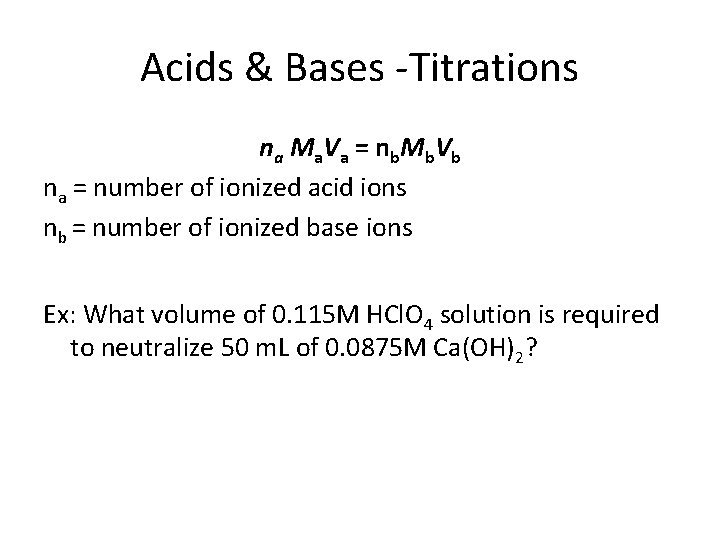
Acids & Bases -Titrations n a M a V a = n b Mb V b na = number of ionized acid ions nb = number of ionized base ions Ex: What volume of 0. 115 M HCl. O 4 solution is required to neutralize 50 m. L of 0. 0875 M Ca(OH)2?

Oxidation- Reduction reactions or Redox Reactions in which one or more electrons are transferred. Oxidation – increase in oxidation state (loss of electrons) - reducing agent Reduction – decrease in oxidation state (gain of electrons) - oxidizing agent 19

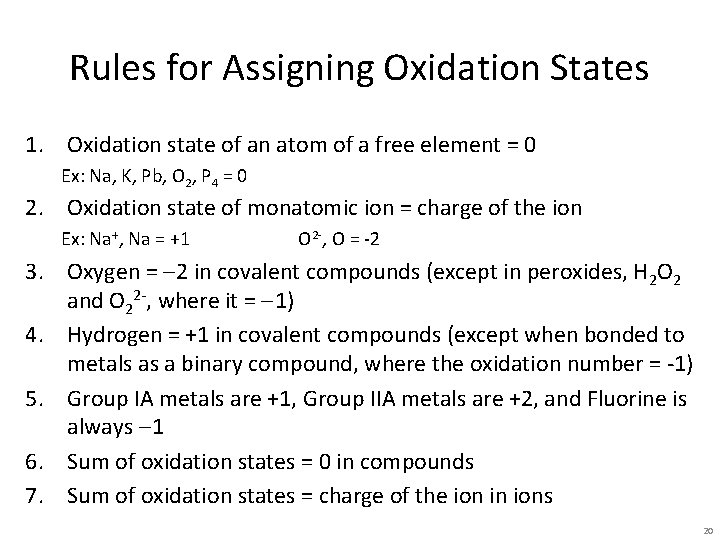
Rules for Assigning Oxidation States 1. Oxidation state of an atom of a free element = 0 Ex: Na, K, Pb, O 2, P 4 = 0 2. Oxidation state of monatomic ion = charge of the ion Ex: Na+, Na = +1 O 2 -, O = -2 3. Oxygen = -2 in covalent compounds (except in peroxides, H 2 O 2 and O 22 -, where it = -1) 4. Hydrogen = +1 in covalent compounds (except when bonded to metals as a binary compound, where the oxidation number = -1) 5. Group IA metals are +1, Group IIA metals are +2, and Fluorine is always -1 6. Sum of oxidation states = 0 in compounds 7. Sum of oxidation states = charge of the ion in ions 20

Find the oxidation number for each of the elements in each of the following compounds: 1. K 2 CO 3 2. HCO 33. Mn. O 4 24. Nal. O 3 21

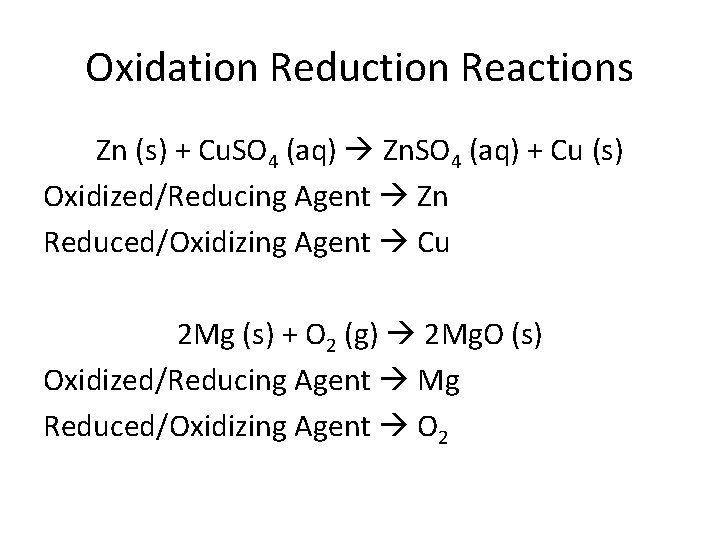
Oxidation Reduction Reactions Zn (s) + Cu. SO 4 (aq) Zn. SO 4 (aq) + Cu (s) Oxidized/Reducing Agent Zn Reduced/Oxidizing Agent Cu 2 Mg (s) + O 2 (g) 2 Mg. O (s) Oxidized/Reducing Agent Mg Reduced/Oxidizing Agent O 2