CHAPTER 4 Nutrients Chapter 4 Nutrients 4 1
















- Slides: 39

CHAPTER 4 Nutrients

Chapter 4 Nutrients 4. 1 The Need for Food 4. 2 Water 4. 3 Carbohydrates 4. 4 Fats 4. 5 Proteins

4. 1 The Need for Food Learning Outcomes After this section, you should be able to: • explain why organisms need food; • state the types of nutrients in food.

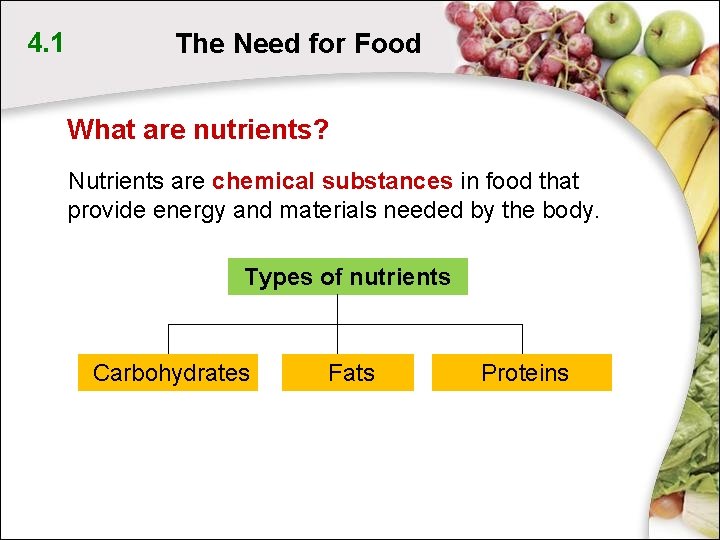
4. 1 The Need for Food Why do we need food? The nutrients in food are needed to: • provide energy • supply raw materials to make new protoplasm • help us stay healthy

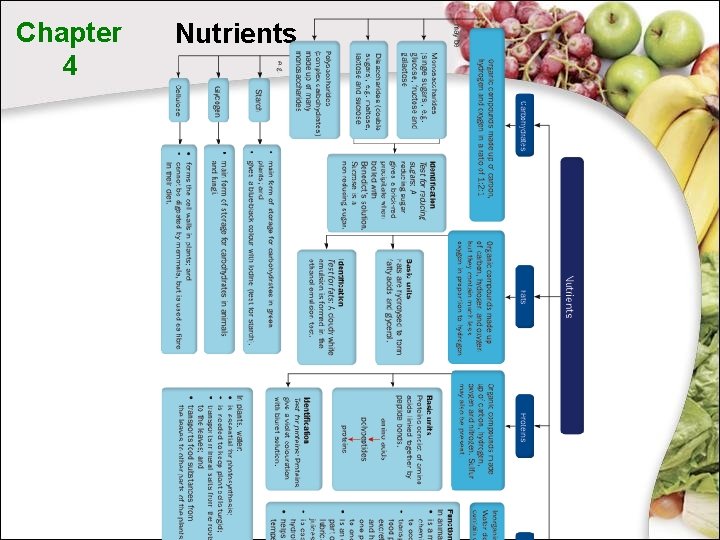
4. 1 The Need for Food What are nutrients? Nutrients are chemical substances in food that provide energy and materials needed by the body. Types of nutrients Carbohydrates Fats Proteins

Chapter 4 Nutrients 4. 1 The Need for Food 4. 2 Water 4. 3 Carbohydrates 4. 4 Fats 4. 5 Proteins

4. 2 Water Learning Outcome After this section, you should be able to: • state the roles of water in living organisms.

4. 2 Water What are the functions of water in humans? Water: • is the solvent in which chemical reactions take place • is an essential component of cells, tissue fluids, digestive juices and blood. • helps control body temperature • helps to transport dissolved substances around the body

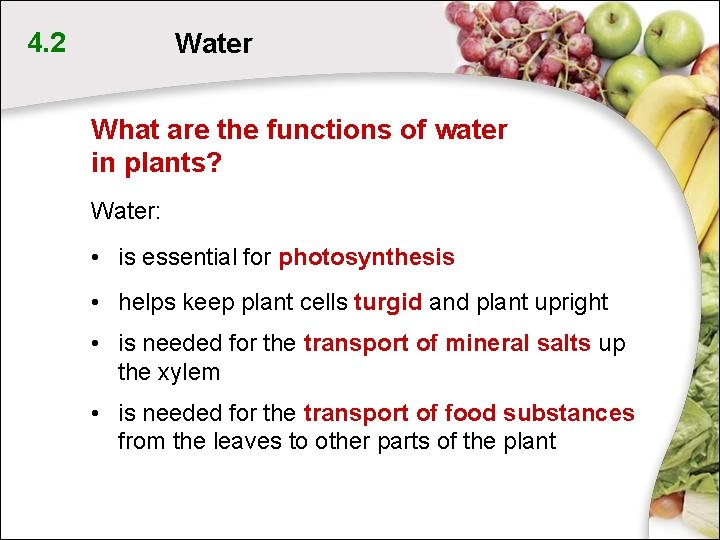
4. 2 Water What are the functions of water in plants? Water: • is essential for photosynthesis • helps keep plant cells turgid and plant upright • is needed for the transport of mineral salts up the xylem • is needed for the transport of food substances from the leaves to other parts of the plant

4. 2 Water How much water do we need? Water is lost from the body when we breathe, sweat or urinate. The water that is lost needs to be replaced. The amount of water needed depends on: • how active the person is • how healthy the person is • the environmental conditions

Chapter 4 Nutrients 4. 1 The Need for Food 4. 2 Water 4. 3 Carbohydrates 4. 4 Fats 4. 5 Proteins

4. 3 Carbohydrates Learning Outcomes After this section, you should be able to: • state the chemical elements in carbohydrates; • understand that glycogen is made from glucose; • investigate for the presence of starch and reducing sugars.

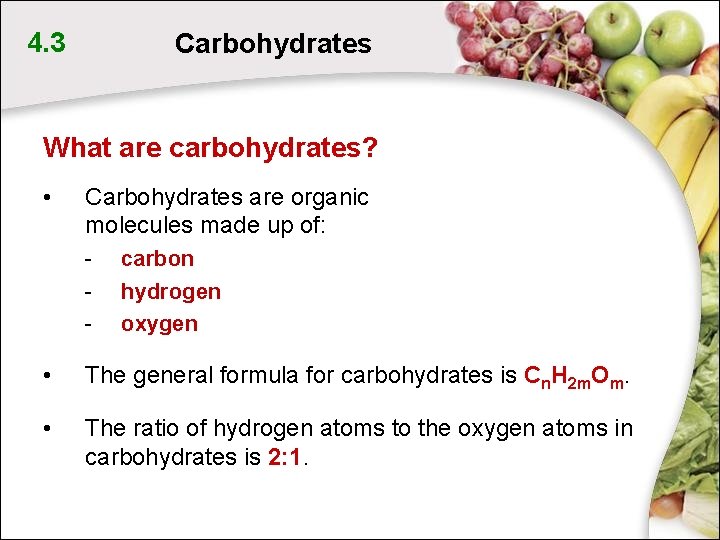
4. 3 Carbohydrates What are carbohydrates? • Carbohydrates are organic molecules made up of: - carbon hydrogen oxygen • The general formula for carbohydrates is Cn. H 2 m. Om. • The ratio of hydrogen atoms to the oxygen atoms in carbohydrates is 2: 1.

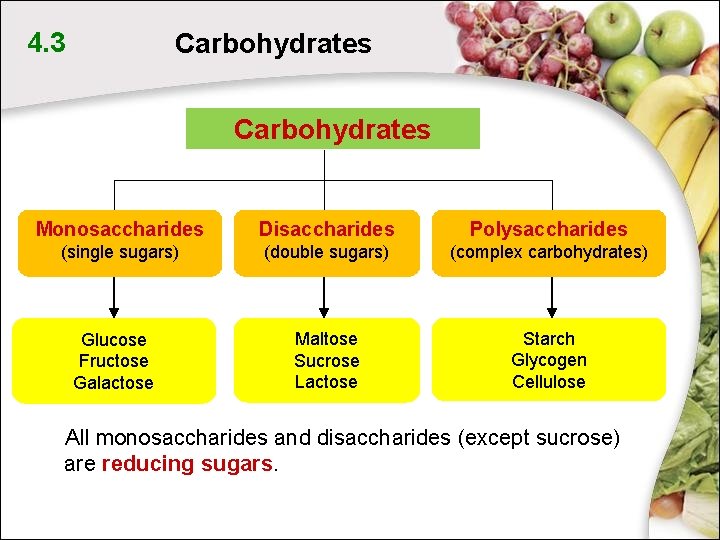
4. 3 Carbohydrates Monosaccharides Disaccharides Polysaccharides (single sugars) (double sugars) (complex carbohydrates) Maltose Sucrose Lactose Starch Glycogen Cellulose Glucose Fructose Galactose All monosaccharides and disaccharides (except sucrose) are reducing sugars.

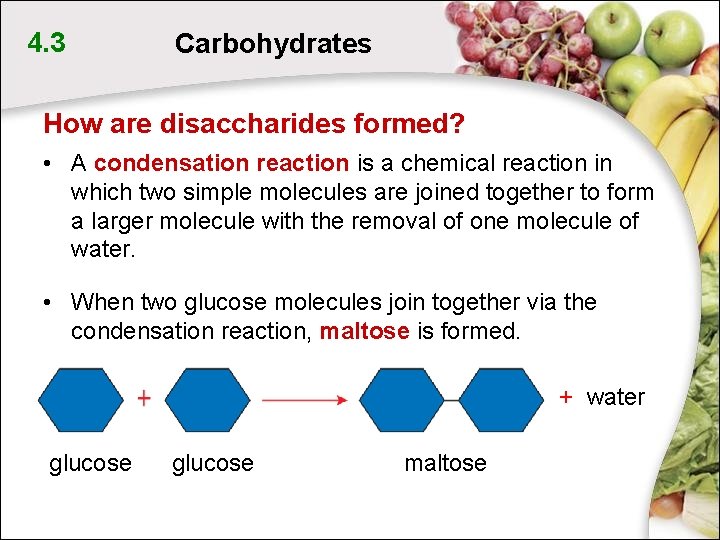
4. 3 Carbohydrates How are disaccharides formed? • A condensation reaction is a chemical reaction in which two simple molecules are joined together to form a larger molecule with the removal of one molecule of water. • When two glucose molecules join together via the condensation reaction, maltose is formed. + water glucose maltose

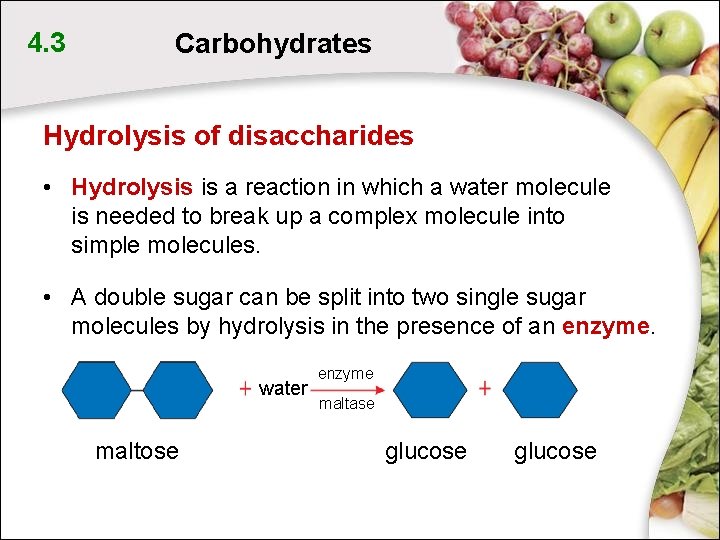
4. 3 Carbohydrates Hydrolysis of disaccharides • Hydrolysis is a reaction in which a water molecule is needed to break up a complex molecule into simple molecules. • A double sugar can be split into two single sugar molecules by hydrolysis in the presence of an enzyme. water maltose enzyme maltase glucose

4. 3 Carbohydrates How can we test for reducing sugars? By carrying out the Benedict’s test 1. Add 2 cm 3 of Benedict’s solution to 2 cm 3 of food sample in a test tube. 2. Shake the mixture. 3. Heat the contents in a boiling water bath for 2 -3 minutes. URL

4. 3 Carbohydrates Benedict’s Test A green, yellow or brick-red precipitate is formed if reducing sugar is present in trace, moderate or large amounts, respectively.

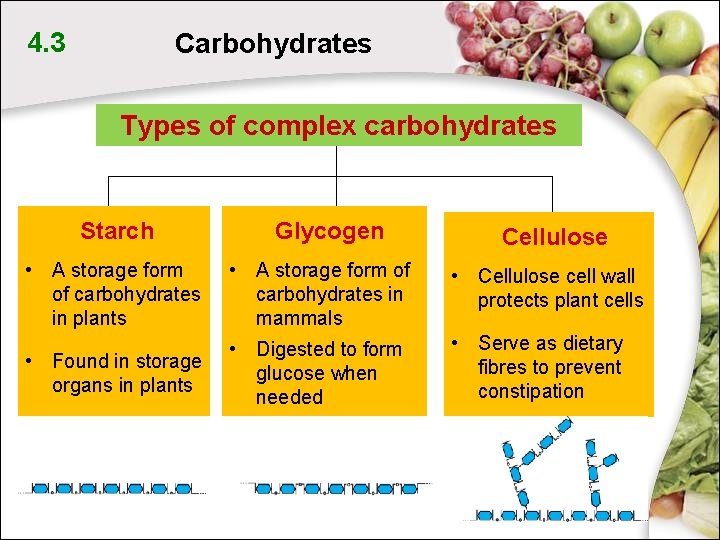
4. 3 Carbohydrates Types of complex carbohydrates Starch Glycogen Cellulose • A storage form of carbohydrates in plants • A storage form of carbohydrates in mammals • Cellulose cell wall protects plant cells • Found in storage organs in plants • Digested to form glucose when needed • Serve as dietary fibres to prevent constipation

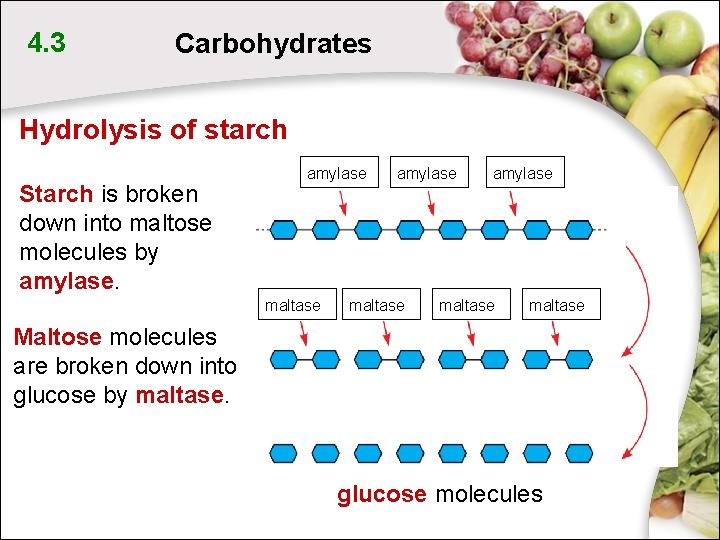
4. 3 Carbohydrates Hydrolysis of starch Starch is broken down into maltose molecules by amylase maltase Maltose molecules are broken down into glucose by maltase. glucose molecules

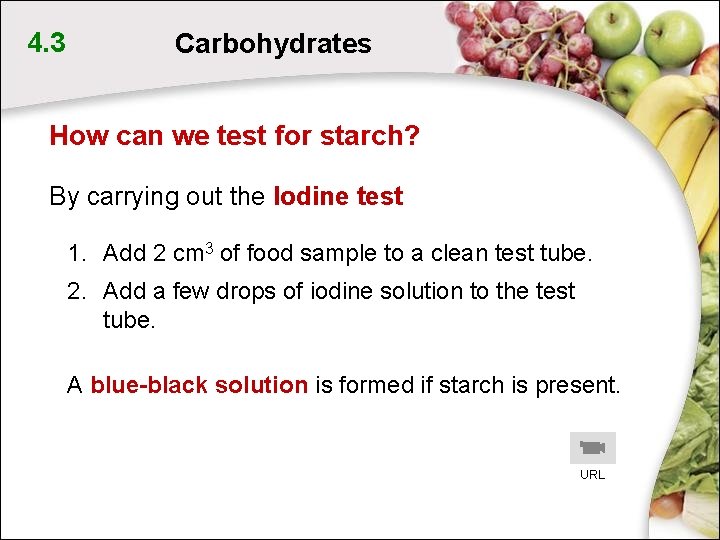
4. 3 Carbohydrates How can we test for starch? By carrying out the Iodine test 1. Add 2 cm 3 of food sample to a clean test tube. 2. Add a few drops of iodine solution to the test tube. A blue-black solution is formed if starch is present. URL

4. 3 Carbohydrates Functions of carbohydrates Carbohydrates are needed: • to provide energy for cell activities • to form supporting structures (e. g. cell wall) • for conversion into other organic compounds (e. g. amino acids and fats) • to form nucleic acids (e. g. DNA) • to synthesise lubricants • to synthesise nectar in flowers

Chapter 4 Nutrients 4. 1 The Need for Food 4. 2 Water 4. 3 Carbohydrates 4. 4 Fats 4. 5 Proteins

4. 4 Fats Learning Outcomes After this section, you should be able to: • state the chemical elements in fats; • understand that fats are made from glycerol and fatty acids; • investigate for the presence of fats.

4. 4 Fats What are fats? • Fats are organic compounds made up of: - carbon hydrogen oxygen • Fats contain much less oxygen in proportion to hydrogen. • The proportions of the elements in fats are not fixed.

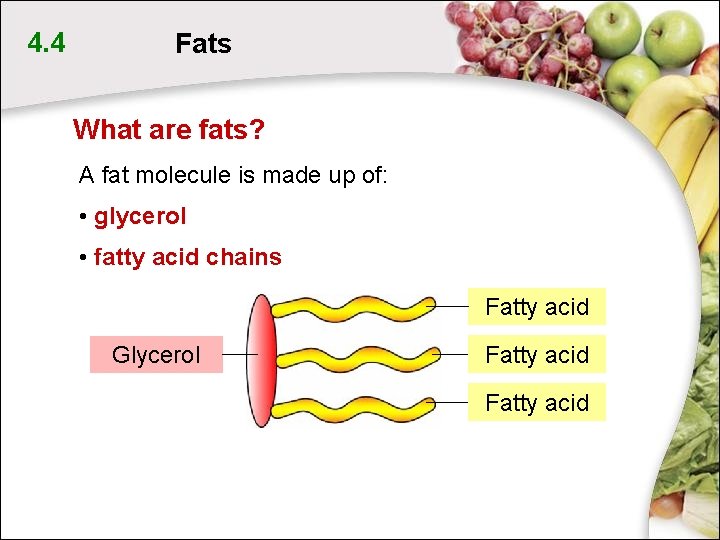
4. 4 Fats What are fats? A fat molecule is made up of: • glycerol • fatty acid chains Fatty acid Glycerol Fatty acid

4. 4 Fats Hydrolysis of fats Fats can be broken down into simpler compounds by hydrolysis. Hydrolysis of fats produces fatty acids and glycerol. enzyme 3 H 2 O Fat molecule 3 water molecules glycerol 3 fatty acid molecules

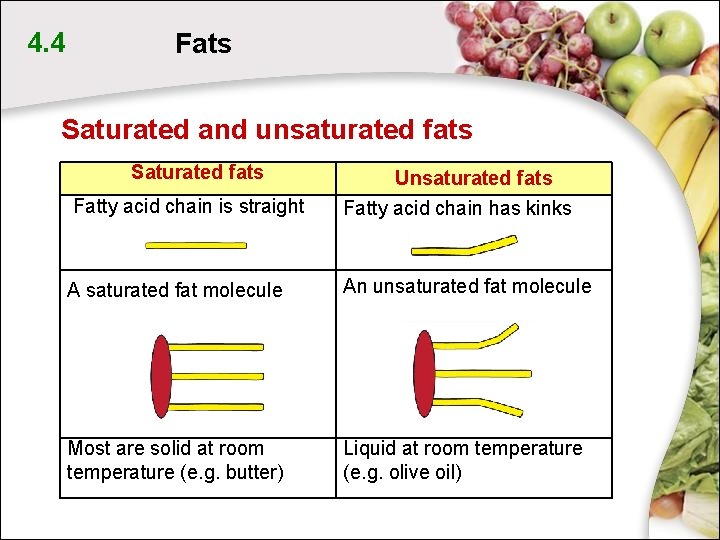
4. 4 Fats Saturated and unsaturated fats Saturated fats Fatty acid chain is straight Unsaturated fats Fatty acid chain has kinks A saturated fat molecule An unsaturated fat molecule Most are solid at room temperature (e. g. butter) Liquid at room temperature (e. g. olive oil)

4. 4 Fats How can we test for fats? By carrying out the Ethanol emulsion test 1. Add 2 cm 3 of ethanol to 2 cm 3 of food sample in a test tube. 2. Shake the contents of the tube vigorously. 3. Decant 3 cm 3 of water into the test tube and shake the mixture. A cloudy white emulsion is formed if fats are present. URL

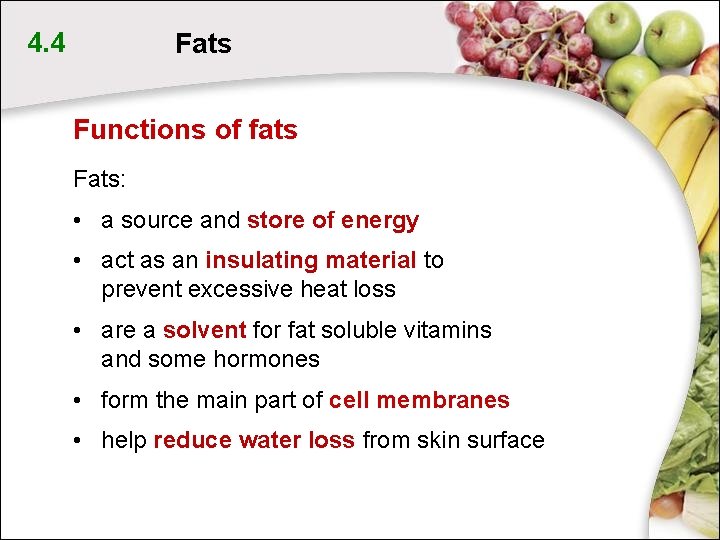
4. 4 Fats Functions of fats Fats: • a source and store of energy • act as an insulating material to prevent excessive heat loss • are a solvent for fat soluble vitamins and some hormones • form the main part of cell membranes • help reduce water loss from skin surface

Chapter 4 Nutrients 4. 1 The Need for Food 4. 2 Water 4. 3 Carbohydrates 4. 4 Fats 4. 5 Proteins

4. 5 Proteins Learning Outcomes After this section, you should be able to: • state the chemical elements in proteins; • understand that polypeptides and proteins are made from amino acids; • investigate for the presence of proteins.

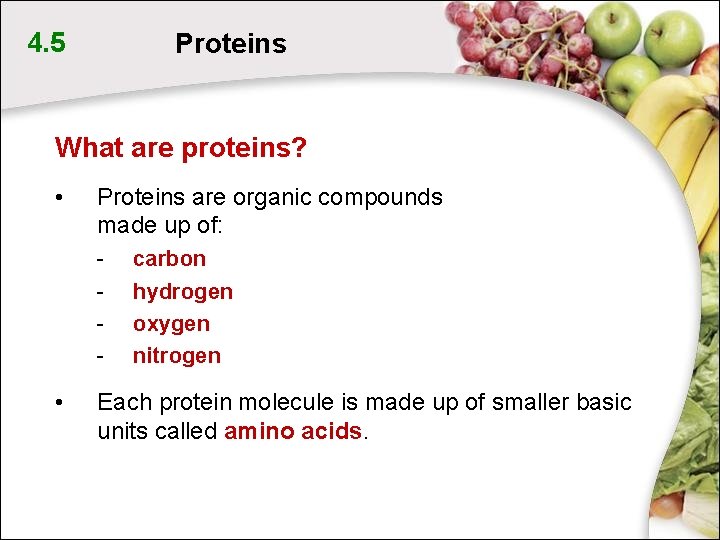
4. 5 Proteins What are proteins? • Proteins are organic compounds made up of: - • carbon hydrogen oxygen nitrogen Each protein molecule is made up of smaller basic units called amino acids.

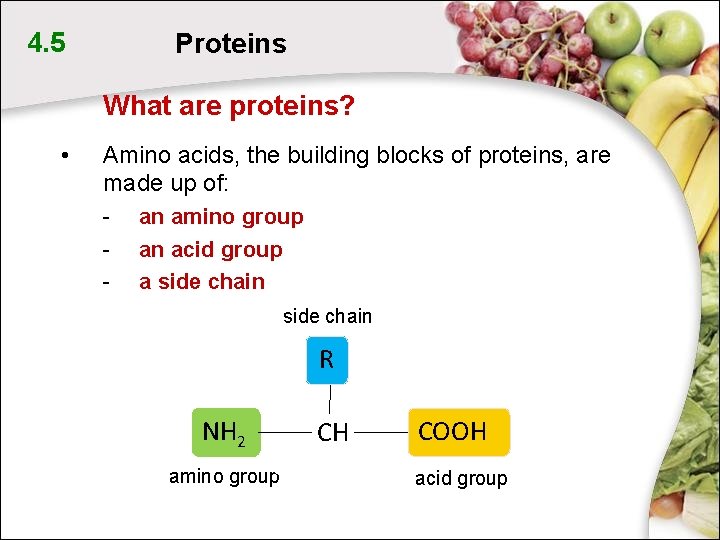
4. 5 Proteins What are proteins? • Amino acids, the building blocks of proteins, are made up of: - an amino group an acid group a side chain R NH 2 amino group CH COOH acid group

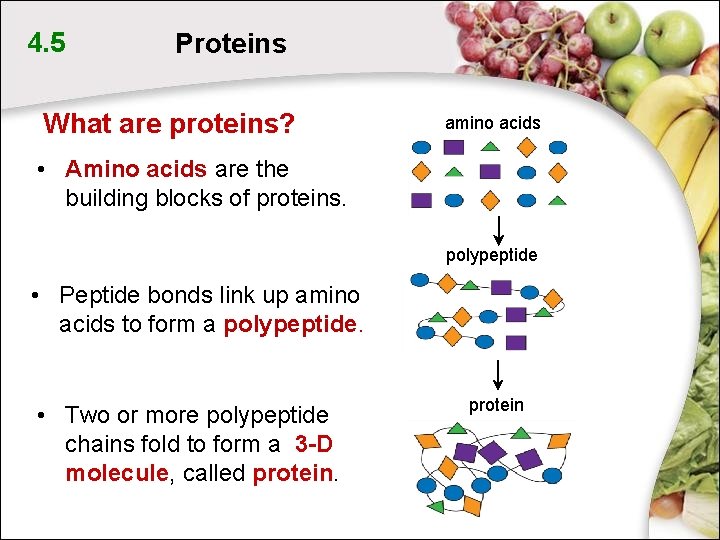
4. 5 Proteins What are proteins? amino acids • Amino acids are the building blocks of proteins. polypeptide • Peptide bonds link up amino acids to form a polypeptide. • Two or more polypeptide chains fold to form a 3 -D molecule, called protein

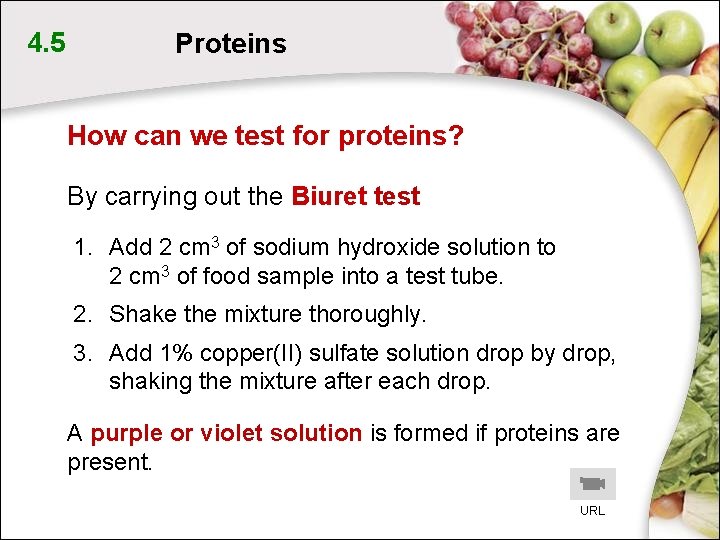
4. 5 Proteins How can we test for proteins? By carrying out the Biuret test 1. Add 2 cm 3 of sodium hydroxide solution to 2 cm 3 of food sample into a test tube. 2. Shake the mixture thoroughly. 3. Add 1% copper(II) sulfate solution drop by drop, shaking the mixture after each drop. A purple or violet solution is formed if proteins are present. URL

4. 5 Proteins Functions of proteins Proteins are needed for: • the synthesis of new protoplasm for growth and repair of worn-out cells • the synthesis of enzymes and some hormones • the synthesis of antibodies

Chapter 4 Nutrients

Chapter 4 Nutrients The URLs are valid as at 15 October 2012.