Chapter 4 Naming and Classifying Compounds Nomenclature Rules

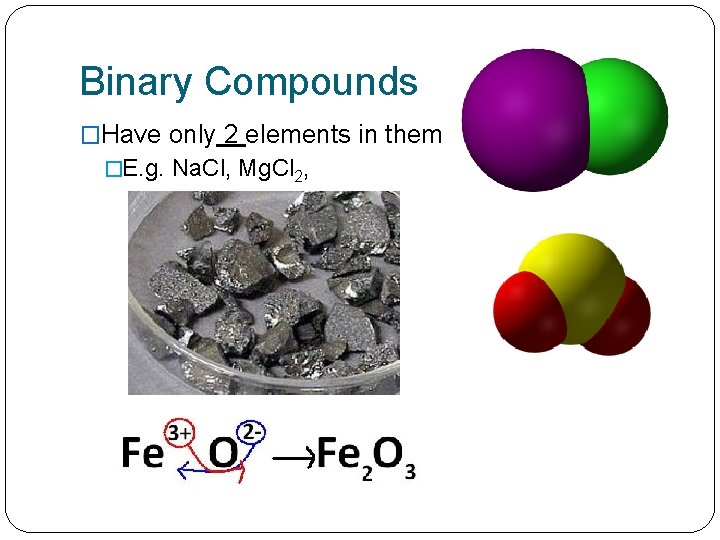







- Slides: 28

Chapter 4 Naming and Classifying Compounds

Nomenclature �Rules for naming substances �Created because too many chemicals had multiple names
Binary Compounds �Have only 2 elements in them �E. g. Na. Cl, Mg. Cl 2,

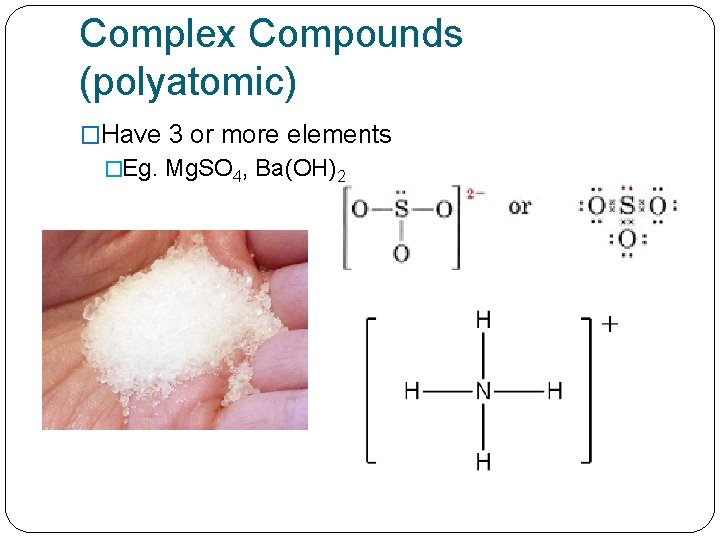
Complex Compounds (polyatomic) �Have 3 or more elements �Eg. Mg. SO 4, Ba(OH)2


Rules for naming compounds �How you name a compound depends on what it is made of �Use the following rules.

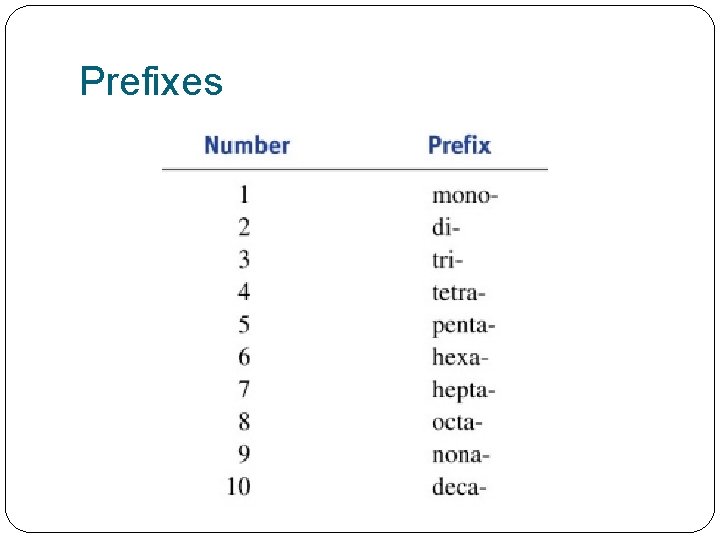
Always �Element 1 keeps its name �Element 2 changes ending to –ide �E. g �oxygen = oxide �Chlorine = chloride �Nitrogen = nitride �Sulphur = sulphide � 2+ atoms = use a prefix �P. 170 #1

Prefixes

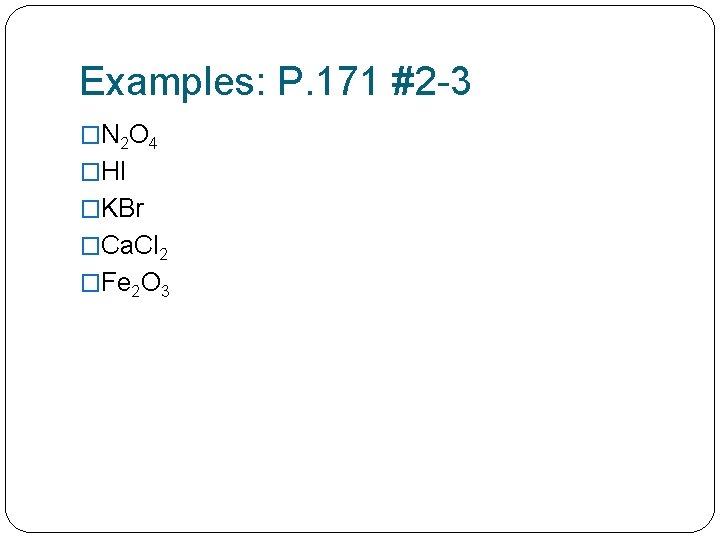
Examples: P. 171 #2 -3 �N 2 O 4 �HI �KBr �Ca. Cl 2 �Fe 2 O 3

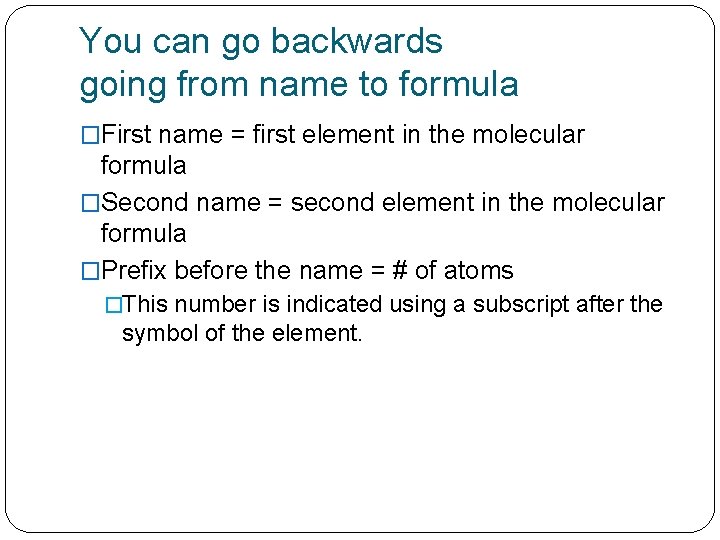
You can go backwards going from name to formula �First name = first element in the molecular formula �Second name = second element in the molecular formula �Prefix before the name = # of atoms �This number is indicated using a subscript after the symbol of the element.

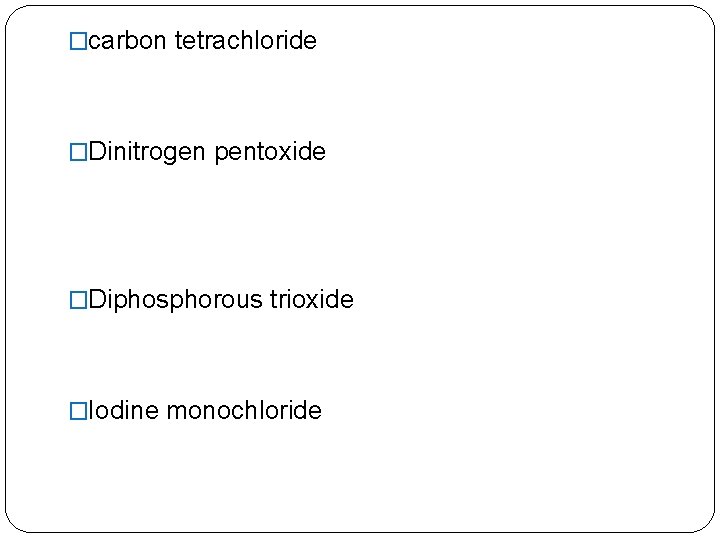
�carbon tetrachloride �Dinitrogen pentoxide �Diphosphorous trioxide �Iodine monochloride

�Magnesium Sulfide �Silver iodide

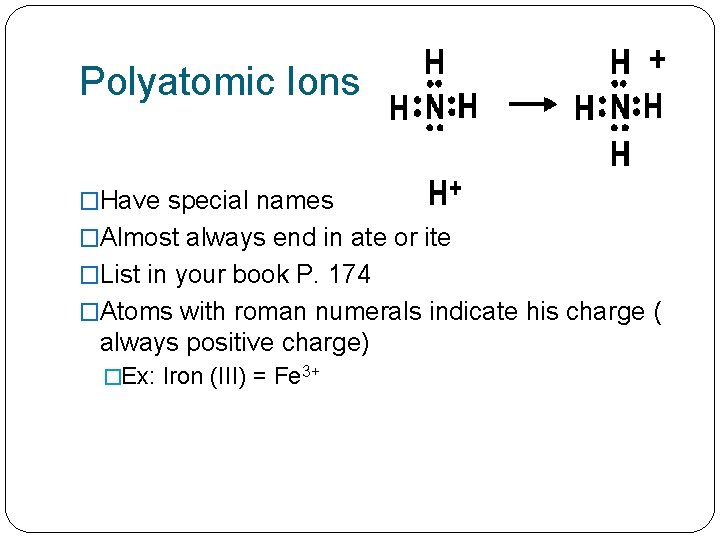
Polyatomic Ions �Have special names �Almost always end in ate or ite �List in your book P. 174 �Atoms with roman numerals indicate his charge ( always positive charge) �Ex: Iron (III) = Fe 3+

Naming compounds that contain at least one polyatomic ion �The compound is divided into two ions (cations and anion) �First name is the Cation �Second name is the Anion �We don’t use prefix

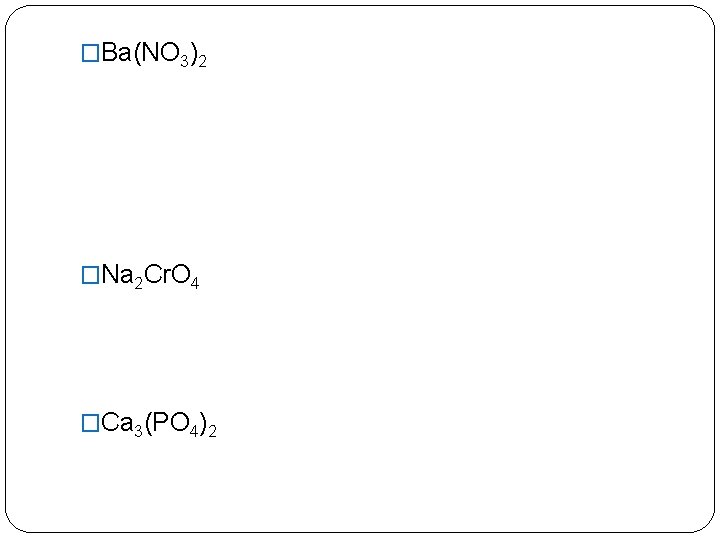
�Ba(NO 3)2 �Na 2 Cr. O 4 �Ca 3(PO 4)2

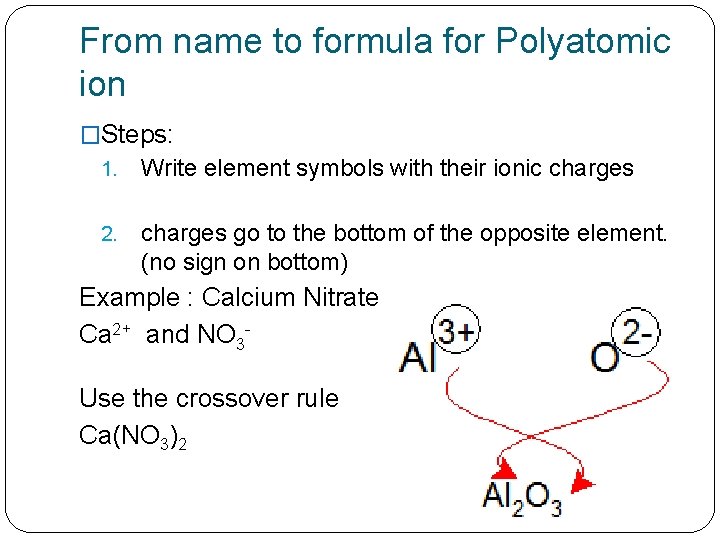
From name to formula for Polyatomic ion �Steps: 1. Write element symbols with their ionic charges 2. charges go to the bottom of the opposite element. (no sign on bottom) Example : Calcium Nitrate Ca 2+ and NO 3 Use the crossover rule Ca(NO 3)2

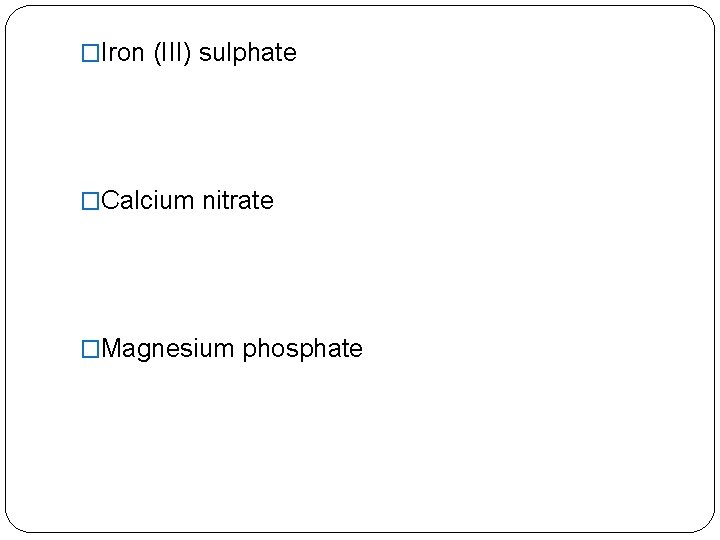
�Iron (III) sulphate �Calcium nitrate �Magnesium phosphate

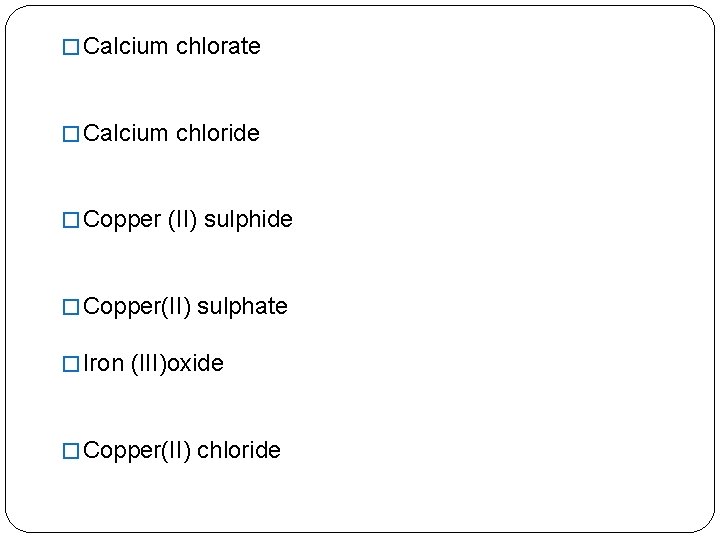
� Calcium chlorate � Calcium chloride � Copper (II) sulphide � Copper(II) sulphate � Iron (III)oxide � Copper(II) chloride

Acids, Bases and Salts �Acids: low p. H, taste sour �Ex. Citrus fruit, vinegar �Bases: high p. H, feel slippery �Soap, milk of magnesium, lye, draino �Salts: neutral p. H �Table salt, road salt, bath salts

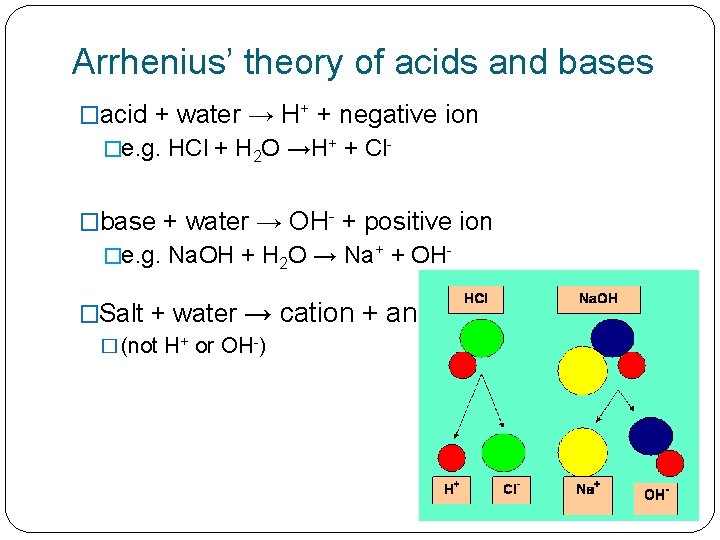
Arrhenius’ theory of acids and bases �acid + water → H+ + negative ion �e. g. HCl + H 2 O →H+ + Cl- �base + water → OH- + positive ion �e. g. Na. OH + H 2 O → Na+ + OH- �Salt + water → � (not H+ or OH-) cation + anion

Dissolution �How things dissolve

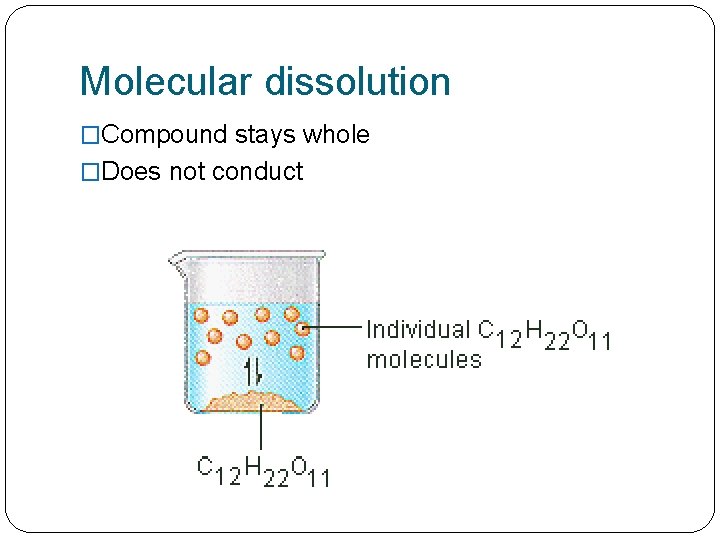
Molecular dissolution �Compound stays whole �Does not conduct

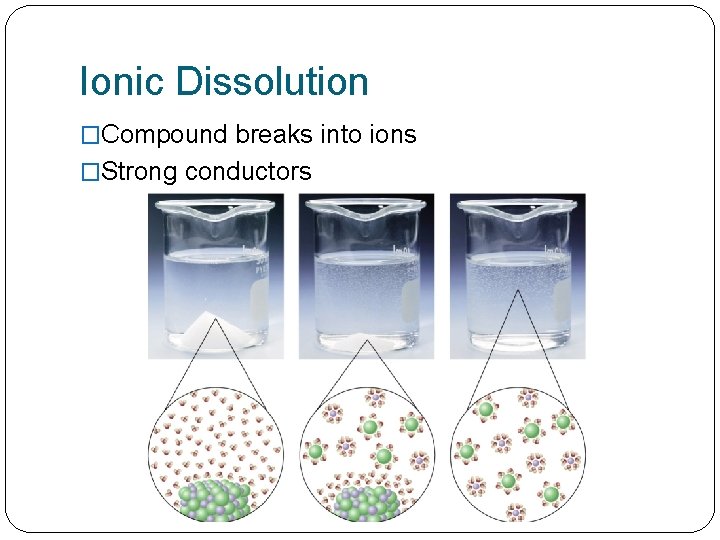
Ionic Dissolution �Compound breaks into ions �Strong conductors

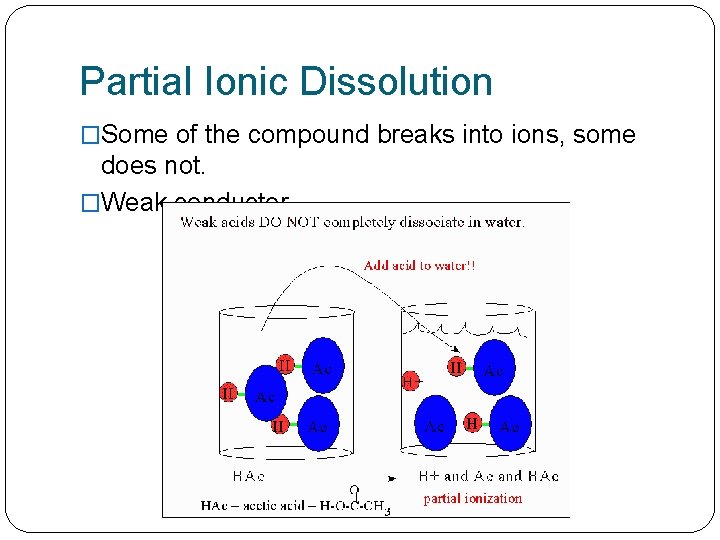
Partial Ionic Dissolution �Some of the compound breaks into ions, some does not. �Weak conductor.

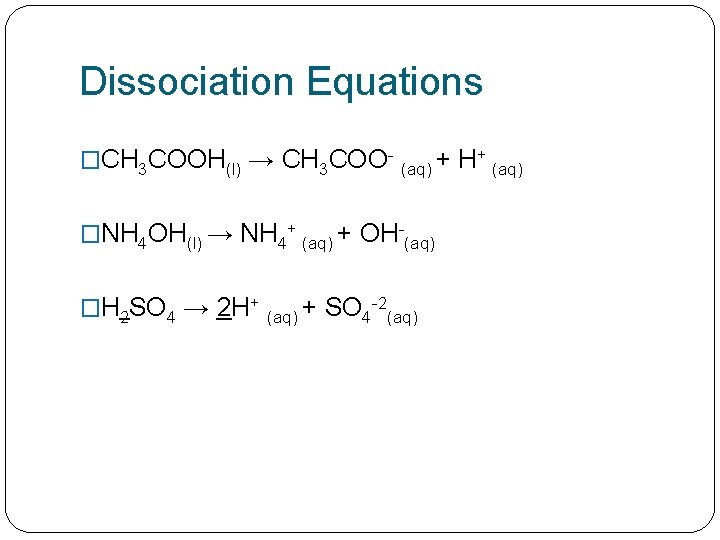
Dissociation Equations �CH 3 COOH(l) → CH 3 COO- (aq) + �NH 4 OH(l) → NH 4+ (aq) + OH-(aq) �H 2 SO 4 → 2 H+ (aq) + SO 4 -2(aq) H+ (aq)

Electrolytes �Ions are electrolytes �They conduct electricity when in water

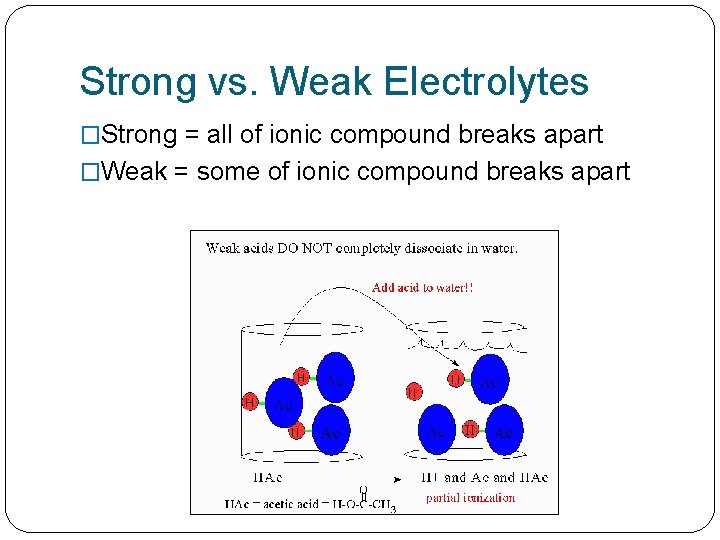
Strong vs. Weak Electrolytes �Strong = all of ionic compound breaks apart �Weak = some of ionic compound breaks apart

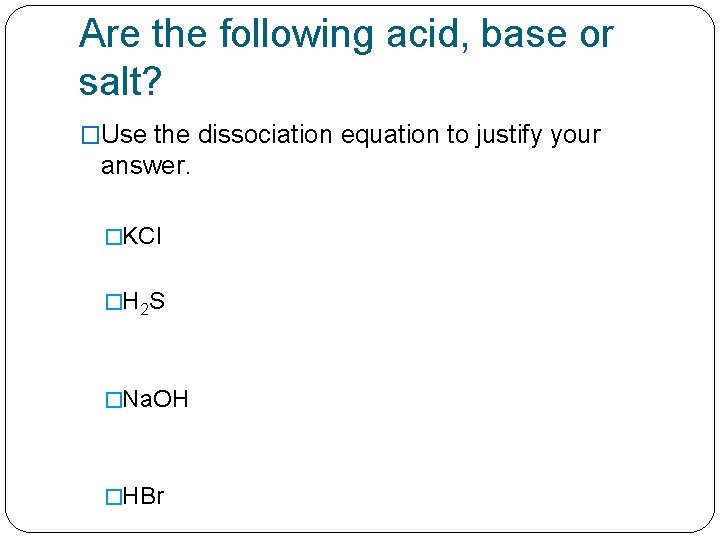
Are the following acid, base or salt? �Use the dissociation equation to justify your answer. �KCl �H 2 S �Na. OH �HBr