Chapter 4 Introduction to Gases Slide 1 Properties






- Slides: 28

Chapter 4 Introduction to Gases Slide 1

Properties of Gases 1. Gases may be compressed. 2. Gases expand to fill their containers. 3. Gases have low densities. Examples are (in units of g/cm 3 at 20°C): a. b. c. d. e. f. Slide 2 Helium = 0. 00017 Air = 0. 0012 Oak (Wood) = 0. 8 Water = 1. 0 Lead = 13. 6 Gold = 19. 3

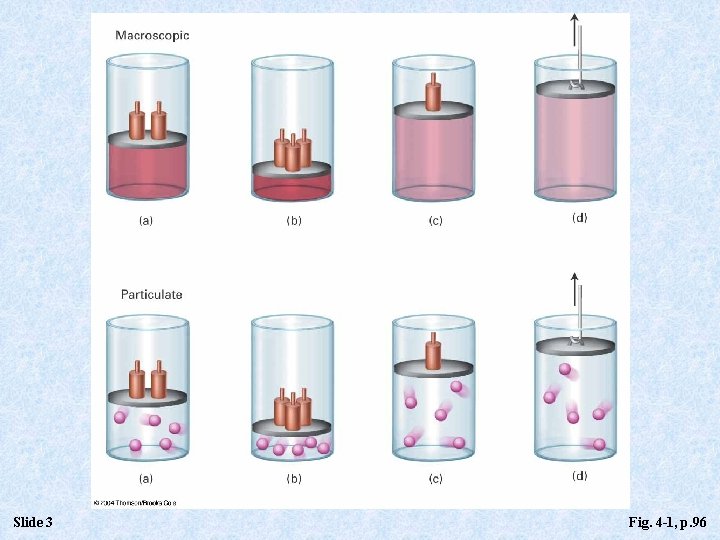
Slide 3 Fig. 4 -1, p. 96

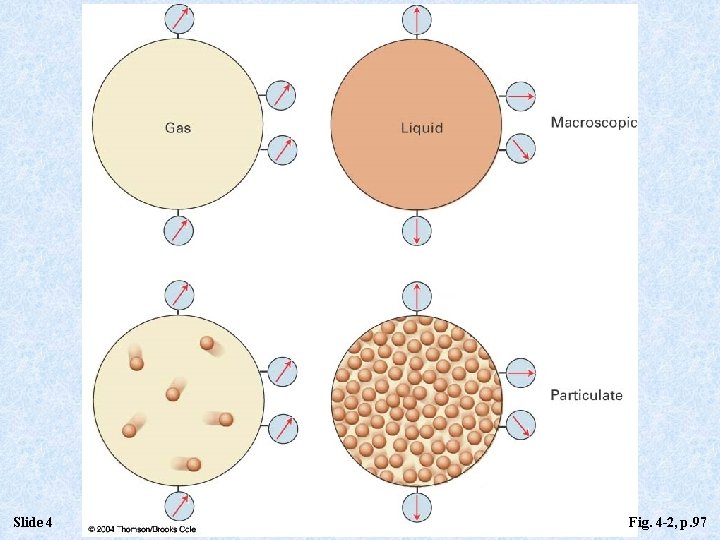
Slide 4 Fig. 4 -2, p. 97

Kinetic Molecular Theory of Gases and the Ideal Gas Model These are a theory and a model used to explain macroscopic (bulk) gas properties and phenomena in terms of the behavior of particles. Slide 5

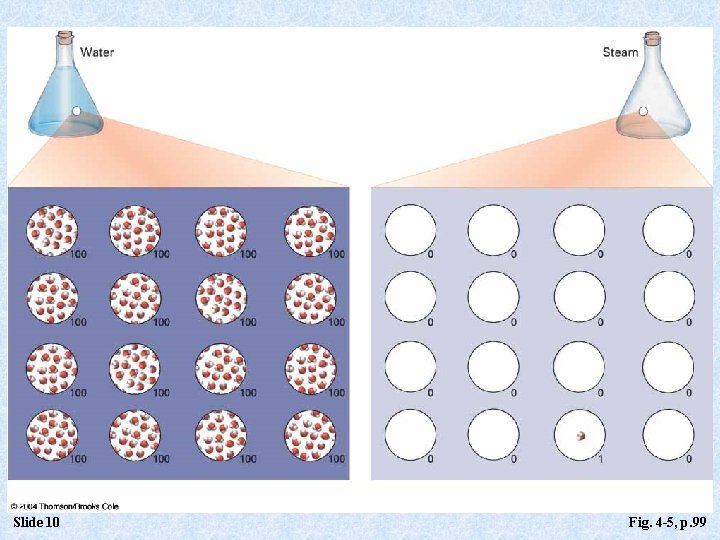
Kinetic Molecular Theory of Gases and the Ideal Gas Model 1. Gases consist of particles in constant motion, traveling in straight lines. 2. Gas particles collide with one another and with container walls without loss of kinetic energy. 3. Gas particles are widely spaced. Consider: a. One gram of liquid water at the normal boiling point occupies 1. 04 cm 3. b. One gram of steam (same number of molecules) occupies 1670 cm 3. Slide 6

Kinetic Molecular Theory of Gases and the Ideal Gas Model 4. The actual volume of particles is negligible compared with the space they occupy. Most of the volume occupied by a gas is empty space. 5. Gas particles behave as independent particles. Attractive forces between the particles are negligible. Slide 7

Slide 8 Fig. 4 -3, p. 98

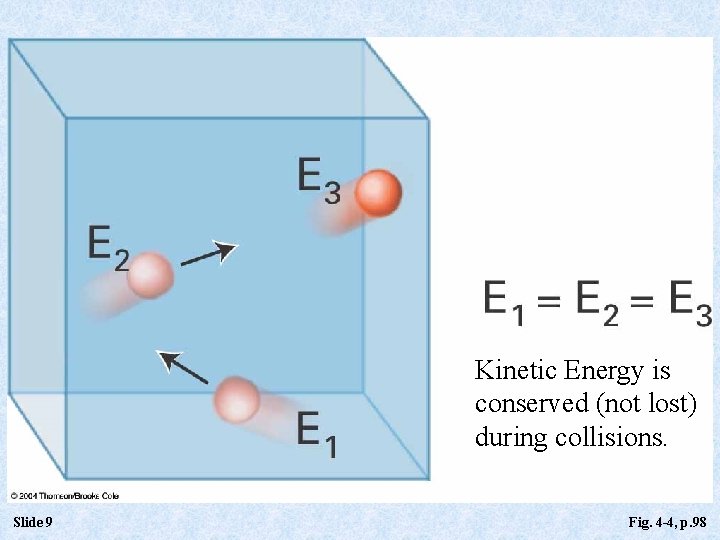
Kinetic Energy is conserved (not lost) during collisions. Slide 9 Fig. 4 -4, p. 98

Slide 10 Fig. 4 -5, p. 99

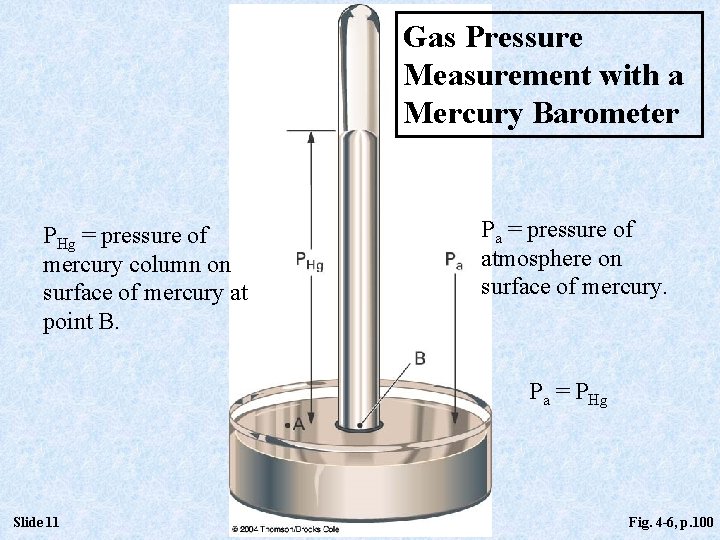
Gas Pressure Measurement with a Mercury Barometer PHg = pressure of mercury column on surface of mercury at point B. Pa = pressure of atmosphere on surface of mercury. Pa = PHg Slide 11 Fig. 4 -6, p. 100

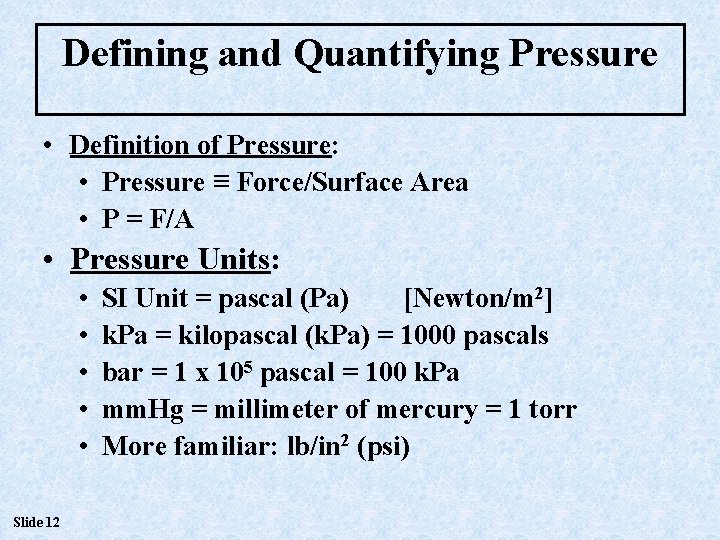
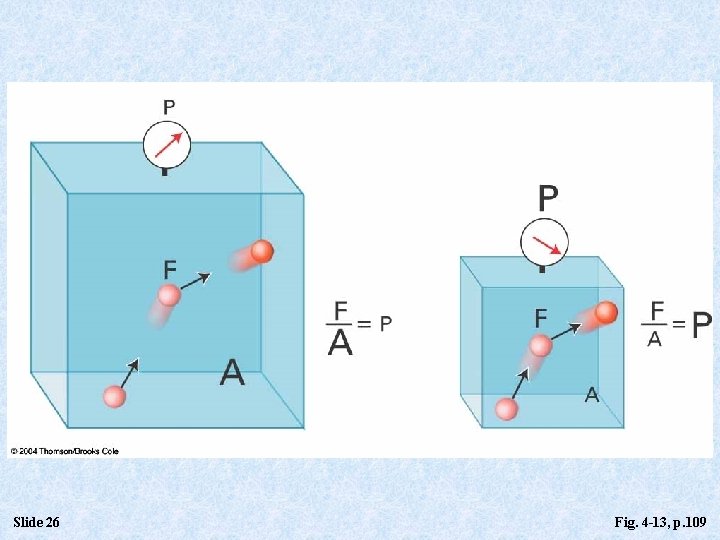
Defining and Quantifying Pressure • Definition of Pressure: • Pressure ≡ Force/Surface Area • P = F/A • Pressure Units: • • • Slide 12 SI Unit = pascal (Pa) [Newton/m 2] k. Pa = kilopascal (k. Pa) = 1000 pascals bar = 1 x 105 pascal = 100 k. Pa mm. Hg = millimeter of mercury = 1 torr More familiar: lb/in 2 (psi)

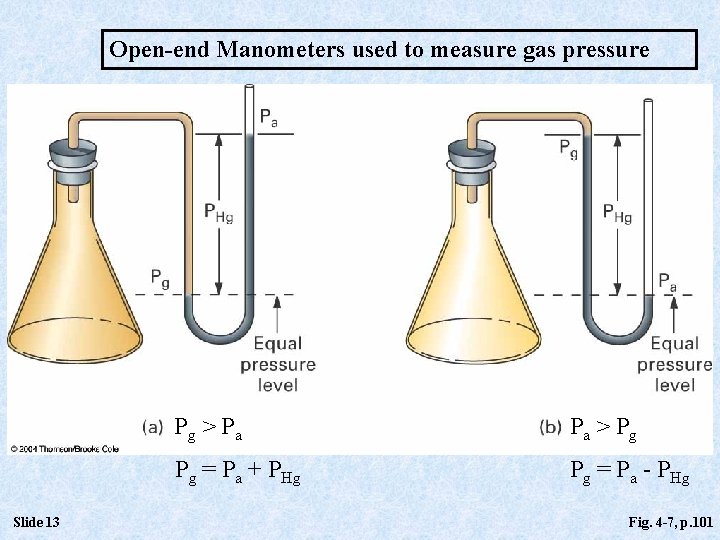
Open-end Manometers used to measure gas pressure Slide 13 Pg > P a Pa > P g Pg = Pa + PHg Pg = Pa - PHg Fig. 4 -7, p. 101

Gas Laws Gas laws are used to relate four measured quantities that describe the state (or condition) of a sample of gas. Those four quantities are: • Volume • Temperature • Pressure • Amount of gas (number of particles) Slide 14

Gas Laws In this chapter, three gas laws will be developed. In a later chapter a fourth gas law will be introduced. The three laws in this chapter are: • Charles’s Law • Boyle’s Law • The Combined Gas Law Slide 15

The Volume-Temperature Law (Charles’s Law) • Charles’s Law: The volume of a fixed quantity of gas at constant pressure is directly proportional to the absolute (Kelvin) temperature. • V T, where the symbol “ ” is read: “is proportional to”, and T is in Kelvin. • V T leads to V = ka. T, where ka is a constant of proportionality. Slide 16

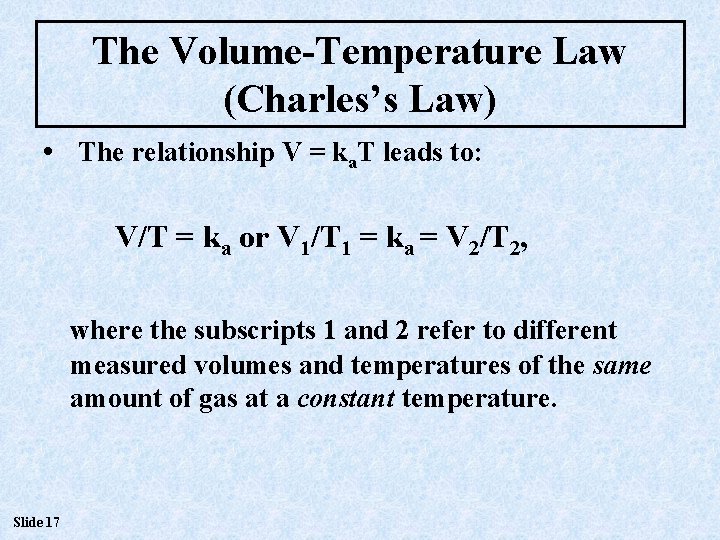
The Volume-Temperature Law (Charles’s Law) • The relationship V = ka. T leads to: V/T = ka or V 1/T 1 = ka = V 2/T 2, where the subscripts 1 and 2 refer to different measured volumes and temperatures of the same amount of gas at a constant temperature. Slide 17

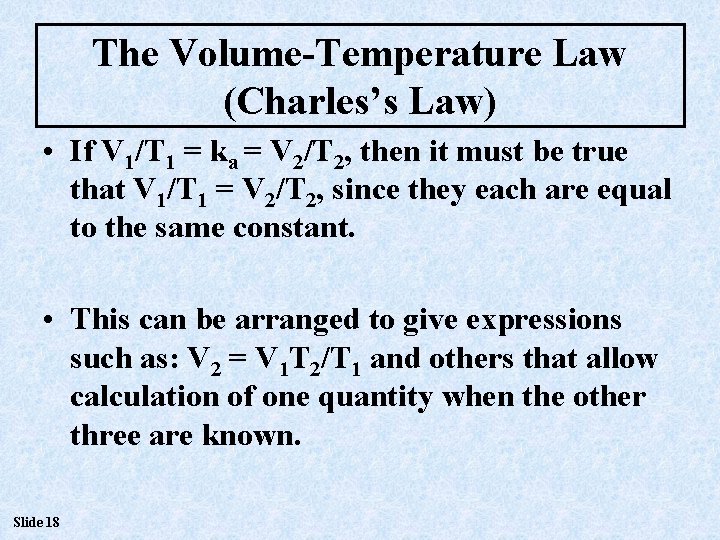
The Volume-Temperature Law (Charles’s Law) • If V 1/T 1 = ka = V 2/T 2, then it must be true that V 1/T 1 = V 2/T 2, since they each are equal to the same constant. • This can be arranged to give expressions such as: V 2 = V 1 T 2/T 1 and others that allow calculation of one quantity when the other three are known. Slide 18

Slide 19 Fig. 4 -9 a, p. 105

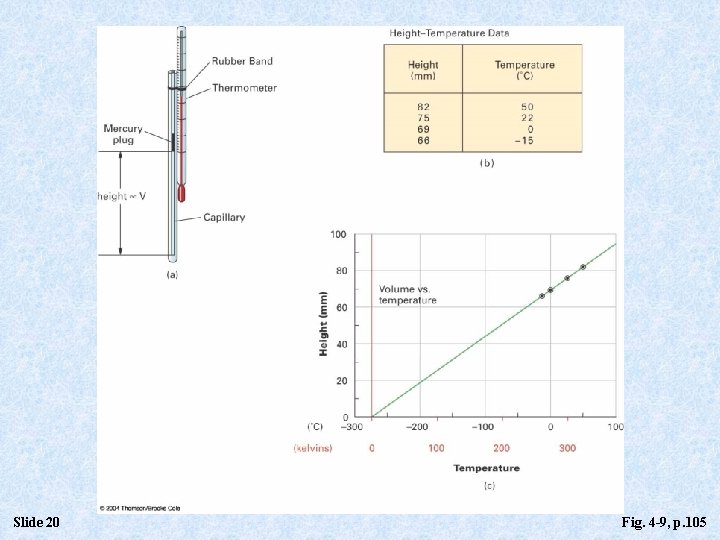
Slide 20 Fig. 4 -9, p. 105

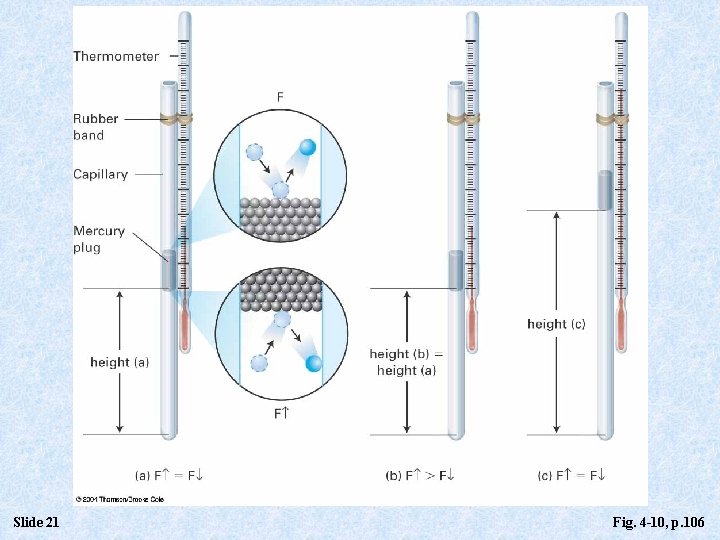
Slide 21 Fig. 4 -10, p. 106

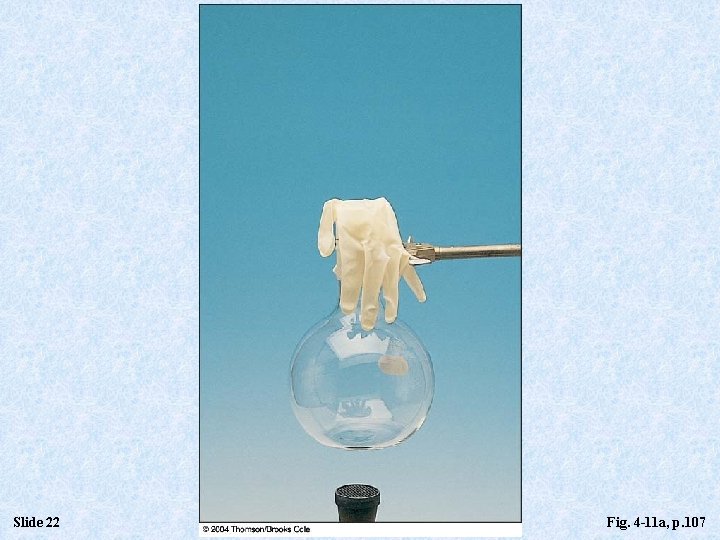
Slide 22 Fig. 4 -11 a, p. 107

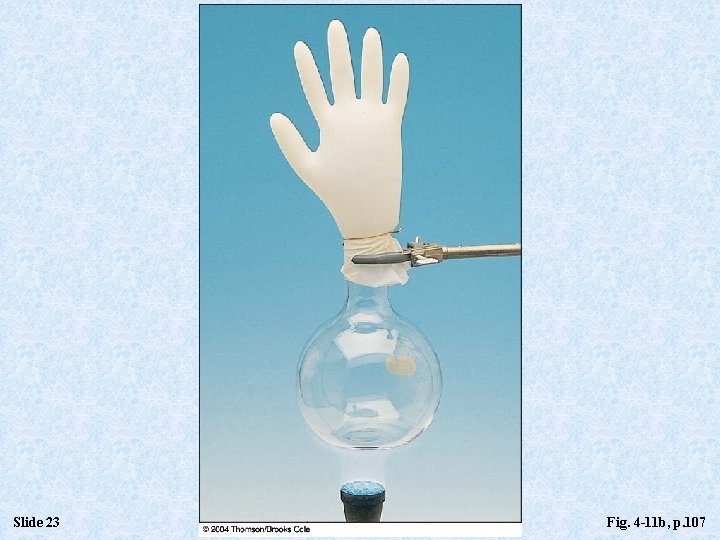
Slide 23 Fig. 4 -11 b, p. 107

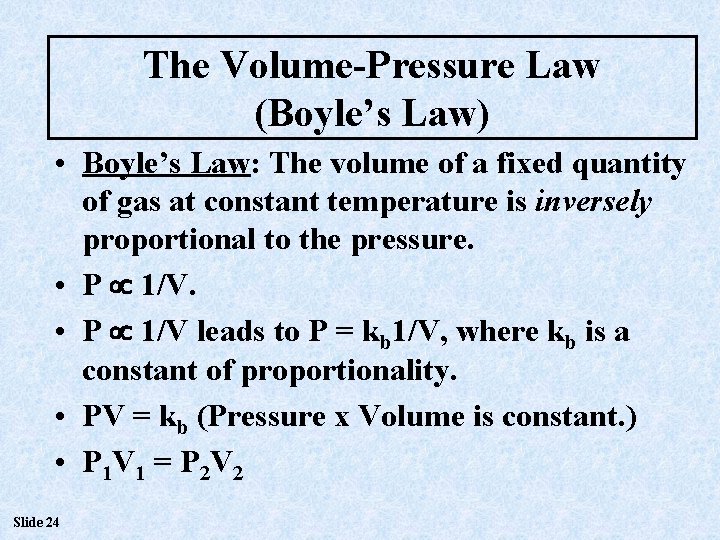
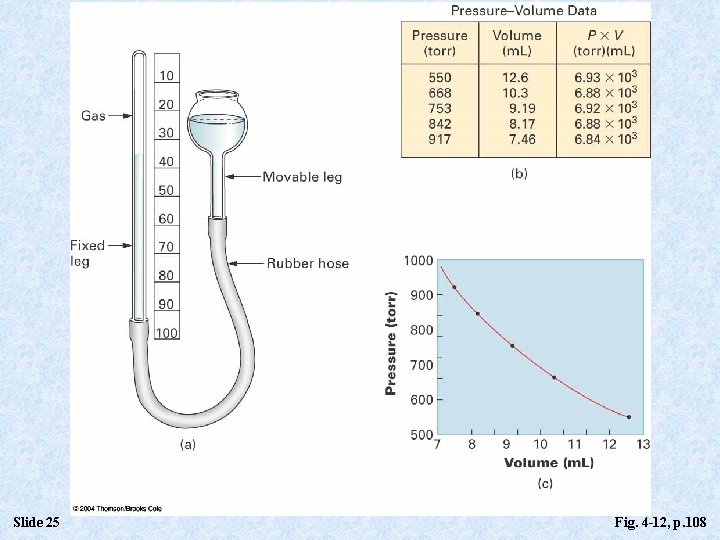
The Volume-Pressure Law (Boyle’s Law) • Boyle’s Law: The volume of a fixed quantity of gas at constant temperature is inversely proportional to the pressure. • P 1/V leads to P = kb 1/V, where kb is a constant of proportionality. • PV = kb (Pressure x Volume is constant. ) • P 1 V 1 = P 2 V 2 Slide 24

Slide 25 Fig. 4 -12, p. 108

Slide 26 Fig. 4 -13, p. 109

Slide 27 p. 110

Slide 28 p. 110