Chapter 4 Elements and Compounds LEARNING OUTCOMES n



- Slides: 28

Chapter 4 Elements and Compounds LEARNING OUTCOMES n. Write formulae to represent ions and molecules n. Write balanced equations including state symbols to represent chemical reactions referred to in the syllabus 1

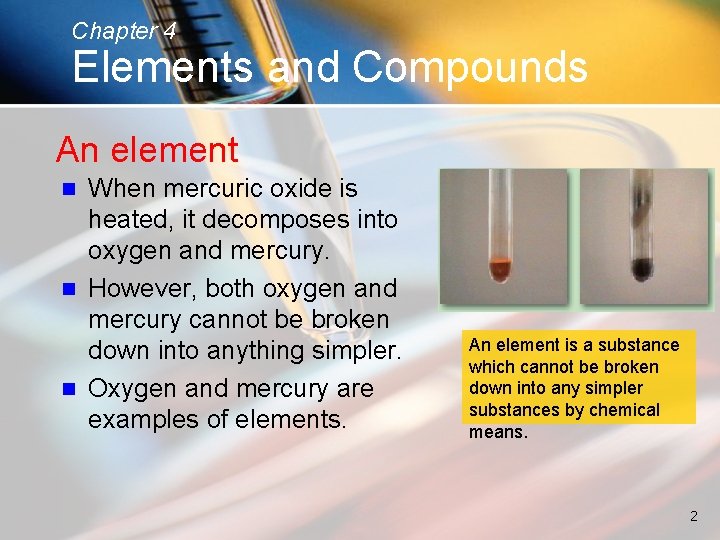
Chapter 4 Elements and Compounds An element When mercuric oxide is heated, it decomposes into oxygen and mercury. n However, both oxygen and mercury cannot be broken down into anything simpler. n Oxygen and mercury are examples of elements. n An element is a substance which cannot be broken down into any simpler substances by chemical means. 2

Chapter 4 Elements and Compounds Elements are the fundamental building blocks of matter in our universe. n There about 92 natural elements and more than 10 man-made elements. n Each element has a name and a chemical symbol. n A list of elements with their symbols is given in the Periodic Table. n The Periodic Table of the Elements http: //www. chemicool. com/ 3

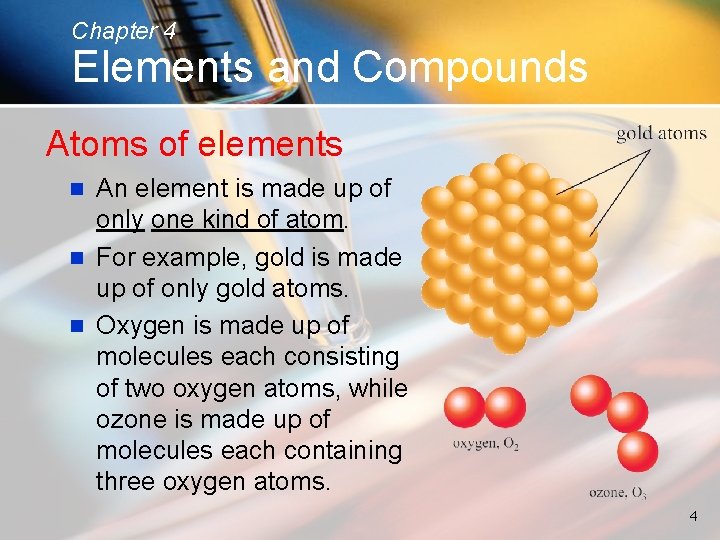
Chapter 4 Elements and Compounds Atoms of elements An element is made up of only one kind of atom. n For example, gold is made up of only gold atoms. n Oxygen is made up of molecules each consisting of two oxygen atoms, while ozone is made up of molecules each containing three oxygen atoms. n 4

Chapter 4 Elements and Compounds Metals and non-metals Elements can be classified into metals and non-metals. n Metals are usually hard and shiny. They are malleable and ductile and are good conductors of heat and electricity. n Non-metals are usually soft and brittle, and are poor conductors of heat and electricity. n There are more metals than nonn Copper: a metal Sulphur: a non- metal 5

Chapter 4 Elements and Compounds Quick check 1 1. 2. 3. “Magnesium is an element”. Explain what this statement means. “A piece of copper can be broken down into very tiny pieces, hence copper is not an element. ” Explain what is wrong with this statement. Give the symbol for each of the following elements. State whether it is a metal or nonmetal. (a) Mercury, (b) Lead, (c) Silver, Solution (d) Chlorine, (e) Strontium, (f) Tungsten. 6

Chapter 4 Elements and Compounds Solution to Quick Check 1 1. 2. 3. Magnesium is an element because it cannot be broken down into simpler substances. Magnesium is made up of magnesium atoms and nothing else. A piece of copper can be broken down into very tiny pieces but each tiny piece of copper is still made up of only copper atoms, hence copper is an element. (a) Mercury: Hg (metal), (b) Lead: Pb (metal), (c) Silver: Ag (metal), (d) Chlorine: Cl (non-metal), (e) Strontium: Sr (metal), (f) tungsten: W (metal). Return 7

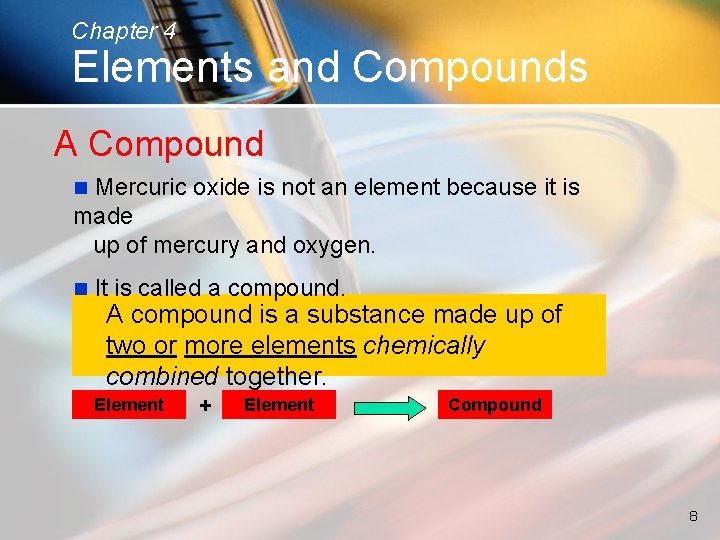
Chapter 4 Elements and Compounds A Compound Mercuric oxide is not an element because it is made up of mercury and oxygen. n n It is called a compound. A compound is a substance made up of two or more elements chemically combined together. Element + Element Compound 8

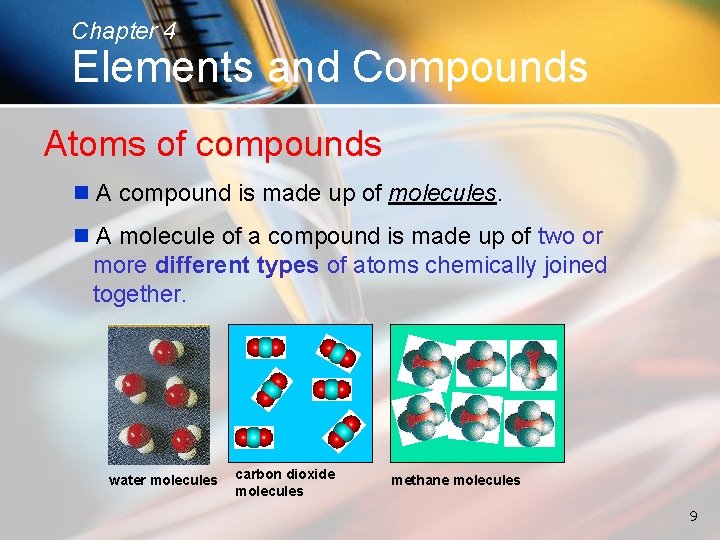
Chapter 4 Elements and Compounds Atoms of compounds n A compound is made up of molecules. n A molecule of a compound is made up of two or more different types of atoms chemically joined together. water molecules carbon dioxide molecules methane molecules 9

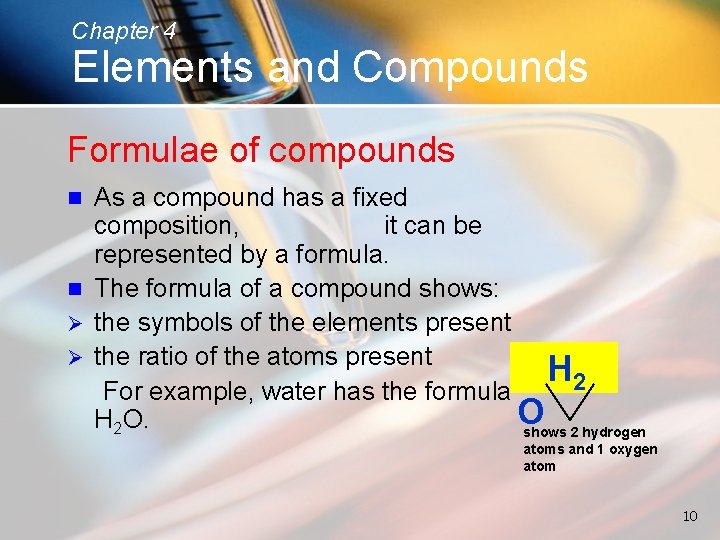
Chapter 4 Elements and Compounds Formulae of compounds As a compound has a fixed composition, it can be represented by a formula. n The formula of a compound shows: Ø the symbols of the elements present Ø the ratio of the atoms present H 2 For example, water has the formula O H 2 O. shows 2 hydrogen n atoms and 1 oxygen atom 10

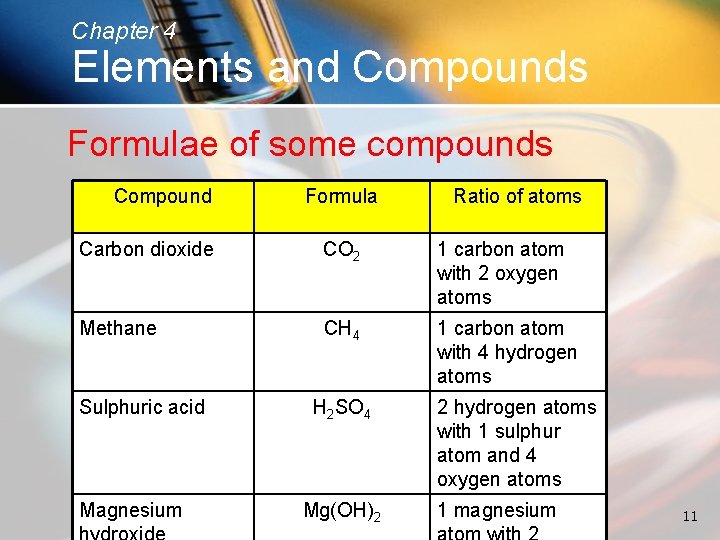
Chapter 4 Elements and Compounds Formulae of some compounds Compound Formula Ratio of atoms Carbon dioxide CO 2 1 carbon atom with 2 oxygen atoms Methane CH 4 1 carbon atom with 4 hydrogen atoms Sulphuric acid Magnesium H 2 SO 4 Mg(OH)2 2 hydrogen atoms with 1 sulphur atom and 4 oxygen atoms 1 magnesium 11

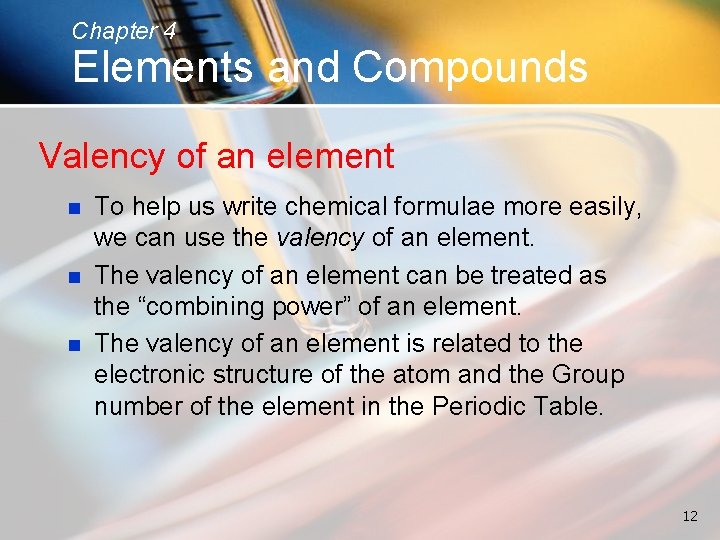
Chapter 4 Elements and Compounds Valency of an element n n n To help us write chemical formulae more easily, we can use the valency of an element. The valency of an element can be treated as the “combining power” of an element. The valency of an element is related to the electronic structure of the atom and the Group number of the element in the Periodic Table. 12

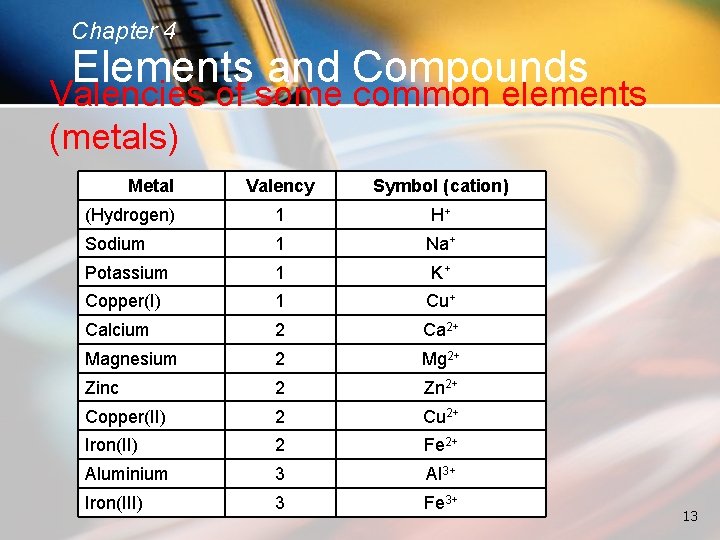
Chapter 4 Elements and Compounds Valencies of some common elements (metals) Metal Valency Symbol (cation) (Hydrogen) 1 H+ Sodium 1 Na+ Potassium 1 K+ Copper(I) 1 Cu+ Calcium 2 Ca 2+ Magnesium 2 Mg 2+ Zinc 2 Zn 2+ Copper(II) 2 Cu 2+ Iron(II) 2 Fe 2+ Aluminium 3 Al 3+ Iron(III) 3 Fe 3+ 13

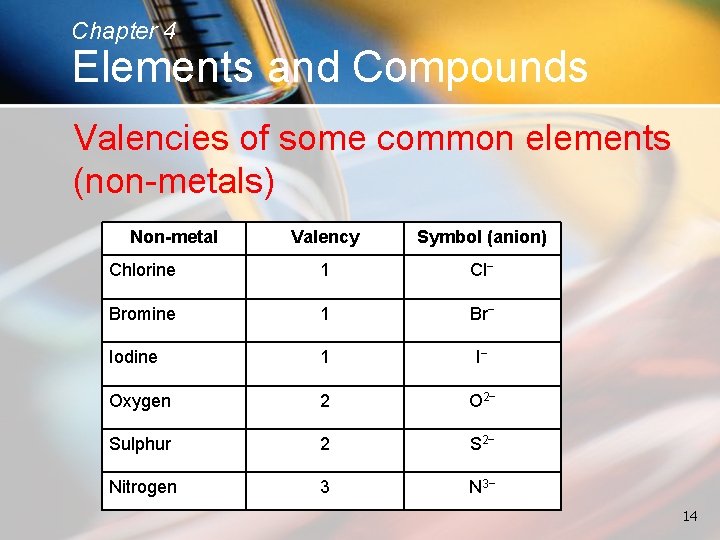
Chapter 4 Elements and Compounds Valencies of some common elements (non-metals) Non-metal Valency Symbol (anion) Chlorine 1 Cl− Bromine 1 Br− Iodine 1 I− Oxygen 2 O 2− Sulphur 2 S 2− Nitrogen 3 N 3− 14

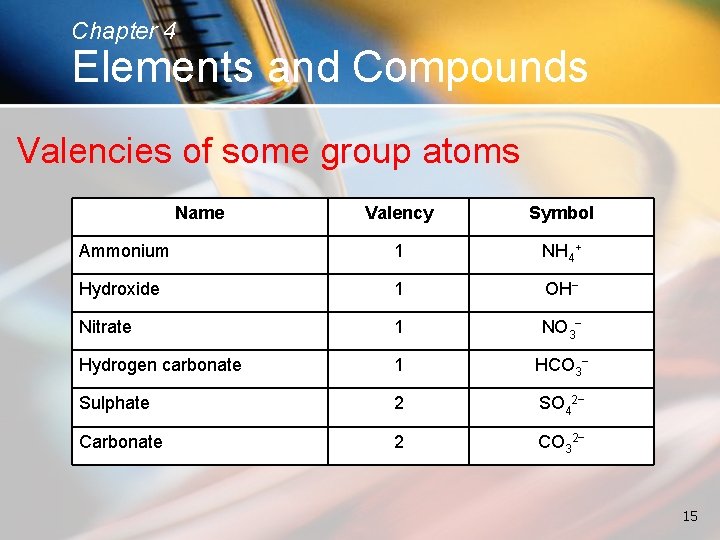
Chapter 4 Elements and Compounds Valencies of some group atoms Name Valency Symbol Ammonium 1 NH 4+ Hydroxide 1 OH− Nitrate 1 NO 3− Hydrogen carbonate 1 HCO 3− Sulphate 2 SO 42− Carbonate 2 CO 32− 15

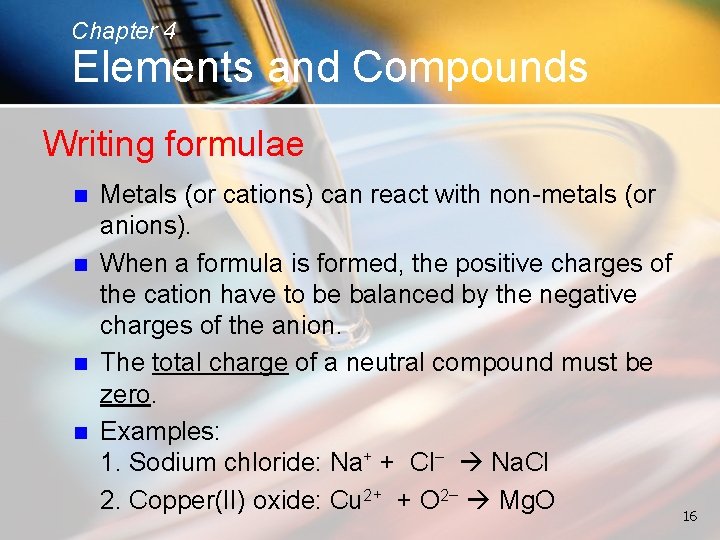
Chapter 4 Elements and Compounds Writing formulae n n Metals (or cations) can react with non-metals (or anions). When a formula is formed, the positive charges of the cation have to be balanced by the negative charges of the anion. The total charge of a neutral compound must be zero. Examples: 1. Sodium chloride: Na+ + Cl− Na. Cl 2. Copper(II) oxide: Cu 2+ + O 2− Mg. O 16

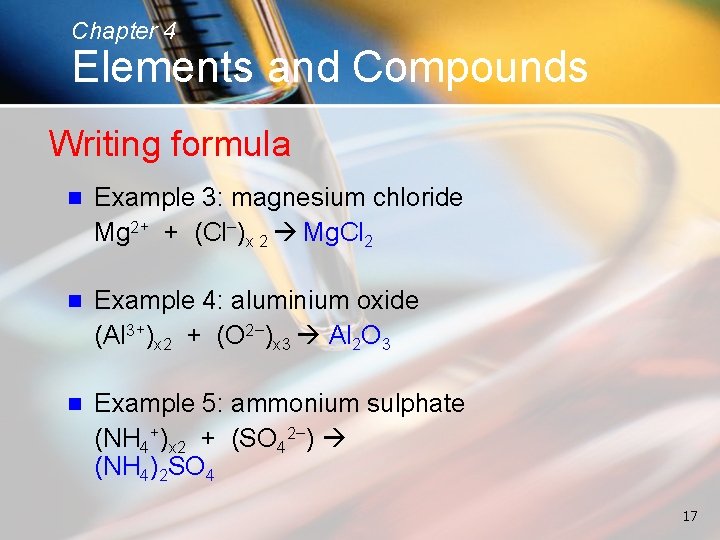
Chapter 4 Elements and Compounds Writing formula n Example 3: magnesium chloride Mg 2+ + (Cl−)x 2 Mg. Cl 2 n Example 4: aluminium oxide (Al 3+)x 2 + (O 2−)x 3 Al 2 O 3 n Example 5: ammonium sulphate (NH 4+)x 2 + (SO 42−) (NH 4)2 SO 4 17

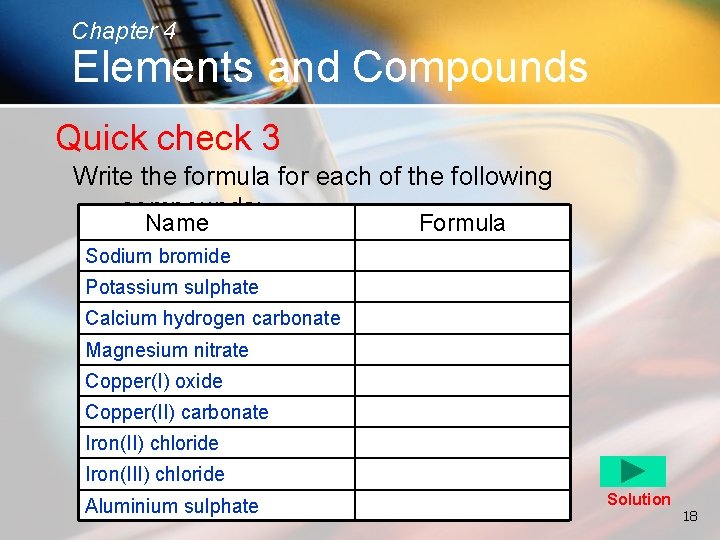
Chapter 4 Elements and Compounds Quick check 3 Write the formula for each of the following compounds: Name Formula Sodium bromide Potassium sulphate Calcium hydrogen carbonate Magnesium nitrate Copper(I) oxide Copper(II) carbonate Iron(II) chloride Iron(III) chloride Aluminium sulphate Solution 18

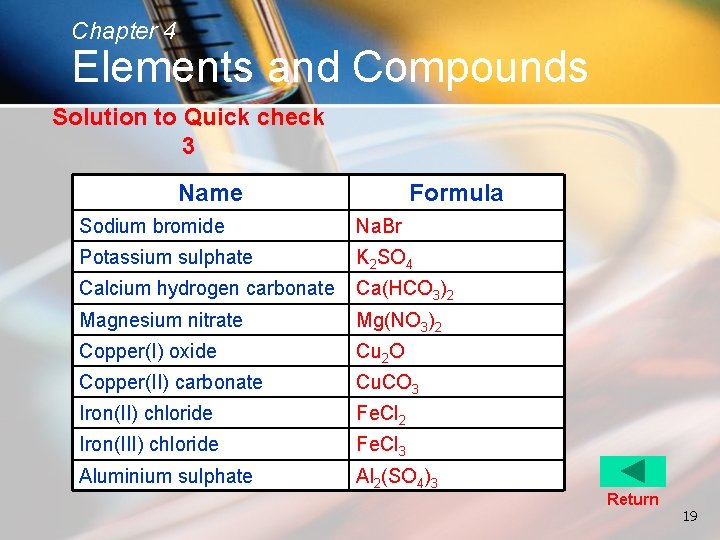
Chapter 4 Elements and Compounds Solution to Quick check 3 Name Formula Sodium bromide Na. Br Potassium sulphate K 2 SO 4 Calcium hydrogen carbonate Ca(HCO 3)2 Magnesium nitrate Mg(NO 3)2 Copper(I) oxide Cu 2 O Copper(II) carbonate Cu. CO 3 Iron(II) chloride Fe. Cl 2 Iron(III) chloride Fe. Cl 3 Aluminium sulphate Al 2(SO 4)3 Return 19

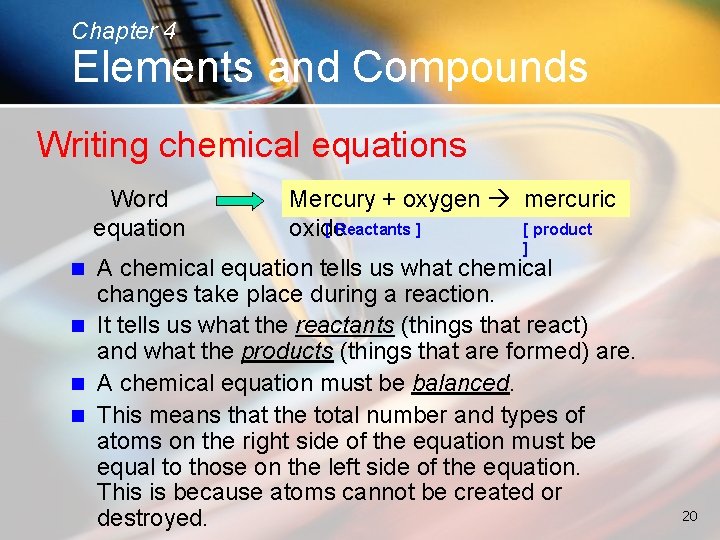
Chapter 4 Elements and Compounds Writing chemical equations Word equation Mercury + oxygen mercuric [ Reactants ] [ product oxide ] A chemical equation tells us what chemical changes take place during a reaction. n It tells us what the reactants (things that react) and what the products (things that are formed) are. n A chemical equation must be balanced. n This means that the total number and types of atoms on the right side of the equation must be equal to those on the left side of the equation. This is because atoms cannot be created or destroyed. n 20

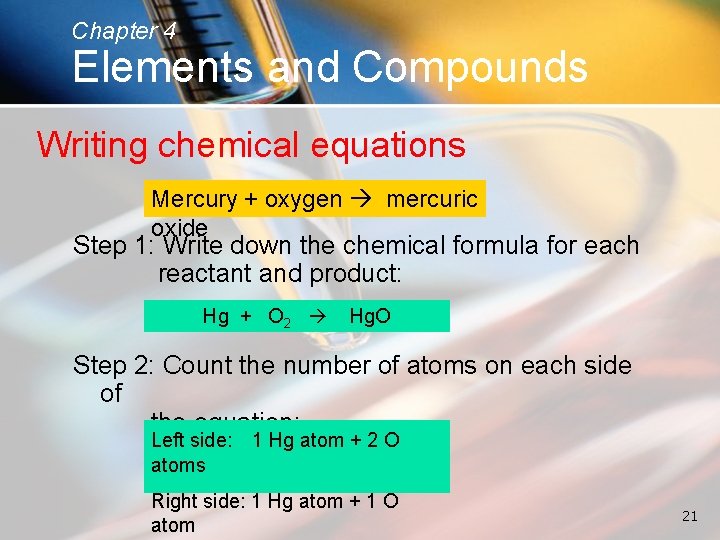
Chapter 4 Elements and Compounds Writing chemical equations Mercury + oxygen mercuric oxide Step 1: Write down the chemical formula for each reactant and product: Hg + O 2 Hg. O Step 2: Count the number of atoms on each side of the equation: Left side: atoms 1 Hg atom + 2 O Right side: 1 Hg atom + 1 O atom 21

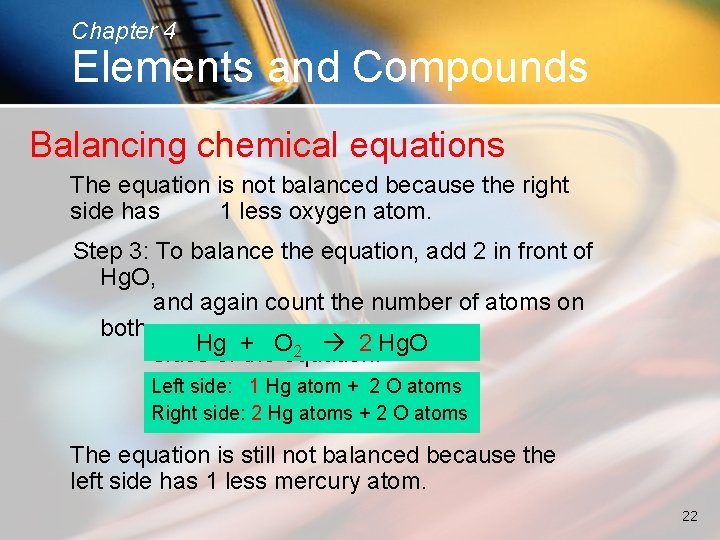
Chapter 4 Elements and Compounds Balancing chemical equations The equation is not balanced because the right side has 1 less oxygen atom. Step 3: To balance the equation, add 2 in front of Hg. O, and again count the number of atoms on both Hgof +the. Oequation: 2 2 Hg. O sides Left side: 1 Hg atom + 2 O atoms Right side: 2 Hg atoms + 2 O atoms The equation is still not balanced because the left side has 1 less mercury atom. 22

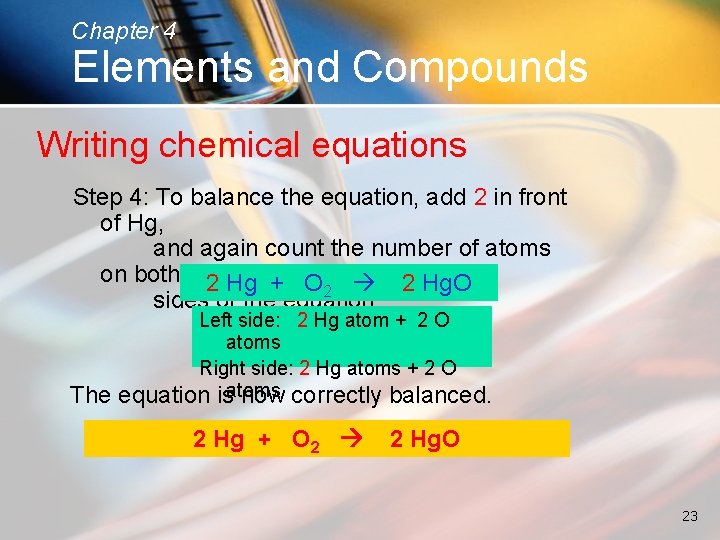
Chapter 4 Elements and Compounds Writing chemical equations Step 4: To balance the equation, add 2 in front of Hg, and again count the number of atoms on both 2 Hg + O 2 Hg. O 2 sides of the equation: The Left side: 2 Hg atom + 2 O atoms Right side: 2 Hg atoms + 2 O equation isatoms now correctly balanced. 2 Hg + O 2 2 Hg. O 23

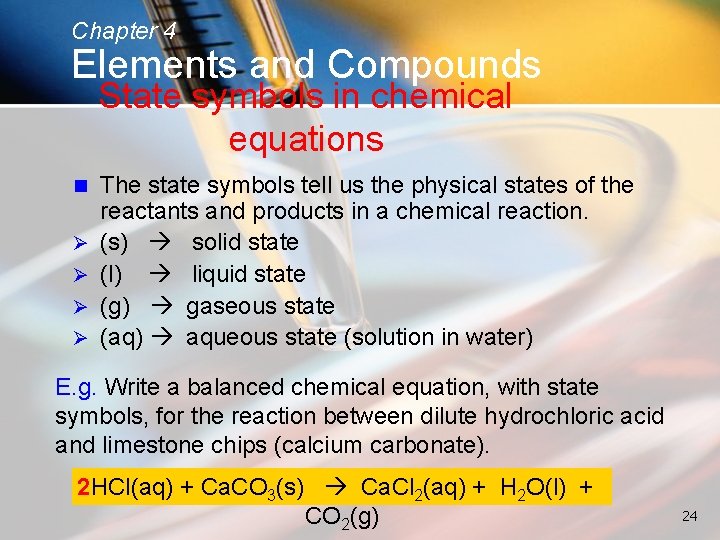
Chapter 4 Elements and Compounds State symbols in chemical equations n Ø Ø The state symbols tell us the physical states of the reactants and products in a chemical reaction. (s) solid state (l) liquid state (g) gaseous state (aq) aqueous state (solution in water) E. g. Write a balanced chemical equation, with state symbols, for the reaction between dilute hydrochloric acid and limestone chips (calcium carbonate). 2 HCl(aq) + Ca. CO 3(s) Ca. Cl 2(aq) + H 2 O(l) + CO 2(g) 24

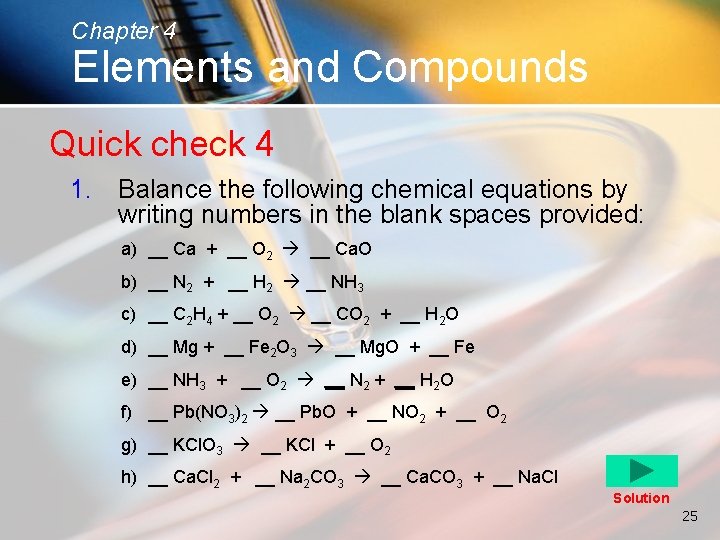
Chapter 4 Elements and Compounds Quick check 4 1. Balance the following chemical equations by writing numbers in the blank spaces provided: a) __ Ca + __ O 2 __ Ca. O b) __ N 2 + __ H 2 __ NH 3 c) __ C 2 H 4 + __ O 2 __ CO 2 + __ H 2 O d) __ Mg + __ Fe 2 O 3 __ Mg. O + __ Fe e) __ NH 3 + __ O 2 __ N 2 + __ H 2 O f) __ Pb(NO 3)2 __ Pb. O + __ NO 2 + __ O 2 g) __ KCl. O 3 __ KCl + __ O 2 h) __ Ca. Cl 2 + __ Na 2 CO 3 __ Ca. CO 3 + __ Na. Cl Solution 25

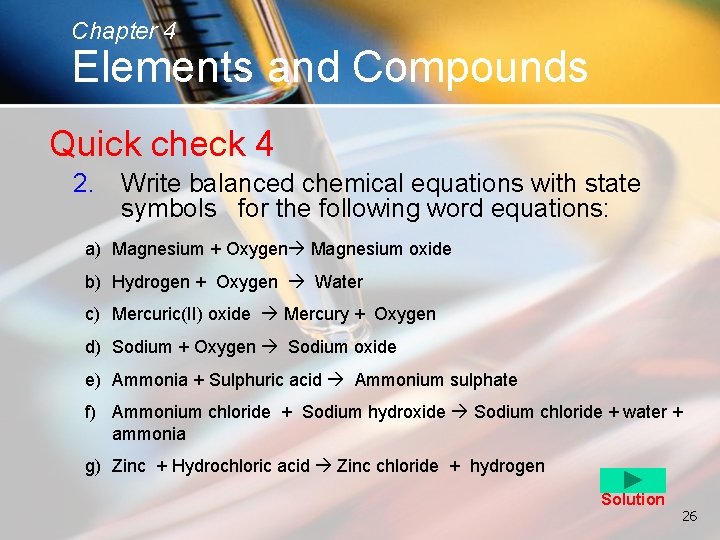
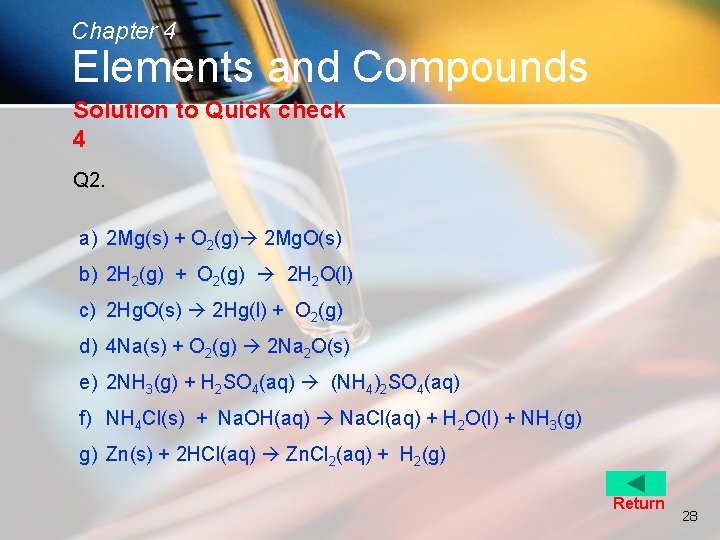
Chapter 4 Elements and Compounds Quick check 4 2. Write balanced chemical equations with state symbols for the following word equations: a) Magnesium + Oxygen Magnesium oxide b) Hydrogen + Oxygen Water c) Mercuric(II) oxide Mercury + Oxygen d) Sodium + Oxygen Sodium oxide e) Ammonia + Sulphuric acid Ammonium sulphate f) Ammonium chloride + Sodium hydroxide Sodium chloride + water + ammonia g) Zinc + Hydrochloric acid Zinc chloride + hydrogen Solution 26

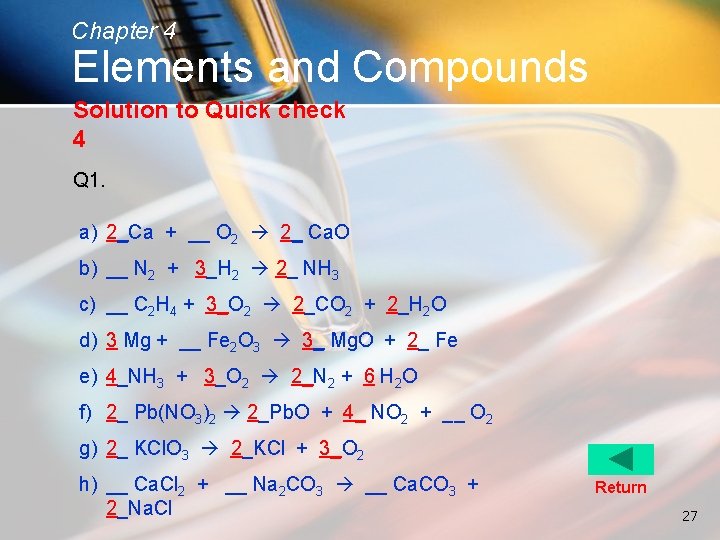
Chapter 4 Elements and Compounds Solution to Quick check 4 Q 1. a) 2_Ca + __ O 2 2_ Ca. O b) __ N 2 + 3_H 2 2_ NH 3 c) __ C 2 H 4 + 3_O 2 2_CO 2 + 2_H 2 O d) 3 Mg + __ Fe 2 O 3 3_ Mg. O + 2_ Fe e) 4_NH 3 + 3_O 2 2_N 2 + 6 H 2 O f) 2_ Pb(NO 3)2 2_Pb. O + 4_ NO 2 + __ O 2 g) 2_ KCl. O 3 2_KCl + 3_O 2 h) __ Ca. Cl 2 + __ Na 2 CO 3 __ Ca. CO 3 + 2_Na. Cl Return 27

Chapter 4 Elements and Compounds Solution to Quick check 4 Q 2. a) 2 Mg(s) + O 2(g) 2 Mg. O(s) b) 2 H 2(g) + O 2(g) 2 H 2 O(l) c) 2 Hg. O(s) 2 Hg(l) + O 2(g) d) 4 Na(s) + O 2(g) 2 Na 2 O(s) e) 2 NH 3(g) + H 2 SO 4(aq) (NH 4)2 SO 4(aq) f) NH 4 Cl(s) + Na. OH(aq) Na. Cl(aq) + H 2 O(l) + NH 3(g) g) Zn(s) + 2 HCl(aq) Zn. Cl 2(aq) + H 2(g) Return 28