Chapter 4 Electronic Structure of Atoms Arrangement of












































- Slides: 69

Chapter 4 Electronic Structure of Atoms (Arrangement of Electrons)

In 1900 Matter and energy were seen as different from each other. n Matter was particles. n Energy was waves, with any frequency. n … that’s what they thought!

Planck investigated blackbody radiation. (emission of light from hot objects). n Max Planck found that the cooling of hot objects couldn’t be explained by viewing energy as a wave. Heated Solids Emit Radiation!!!

* So New Findings n Electrons and light have a dual nature, called the wave-particle model. n Sometimes light behaves like a wave, sometimes light behaves like a particle.

Light is…. n Made up of electromagnetic radiation (radiant energy). n Radiation carries energy through space via electromagnetic waves. n Waves of electric and magnetic fields at right angles to each other.

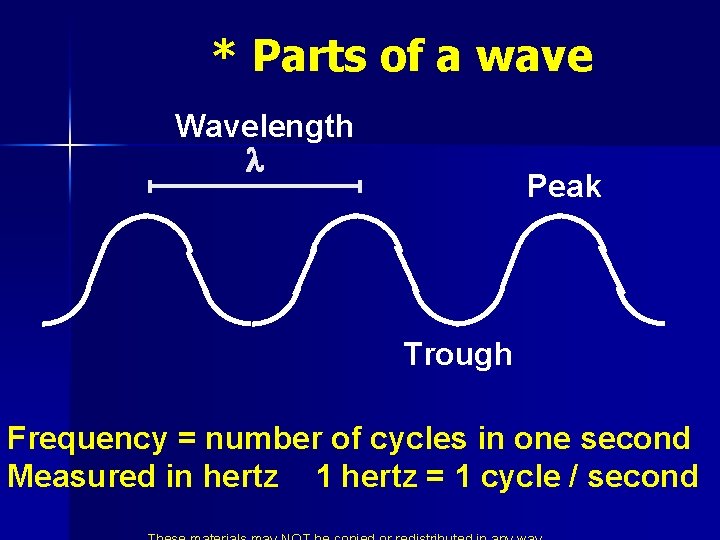
* Parts of a wave Wavelength l Peak Trough Frequency = number of cycles in one second Measured in hertz 1 hertz = 1 cycle / second

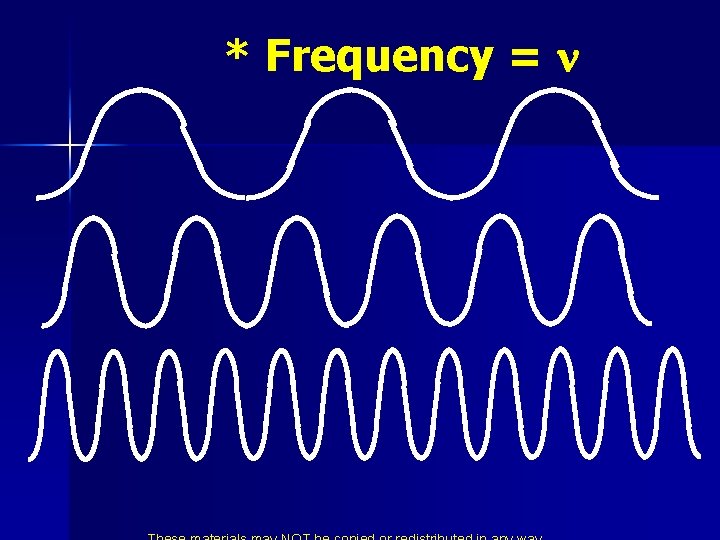
* Frequency = n

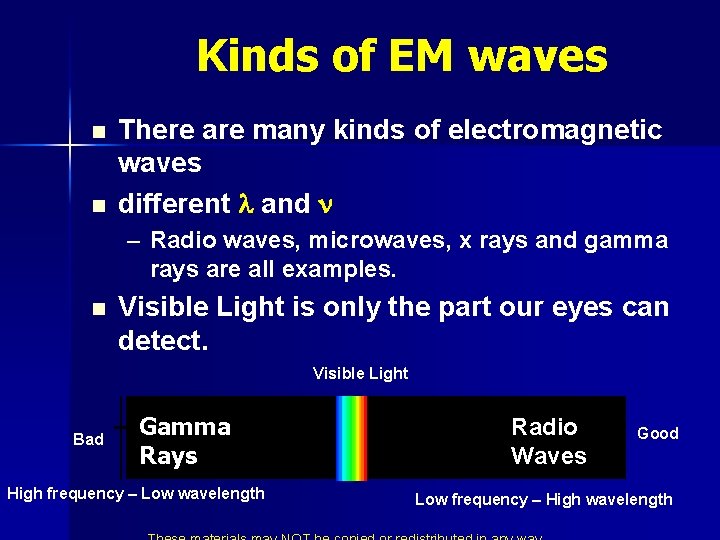
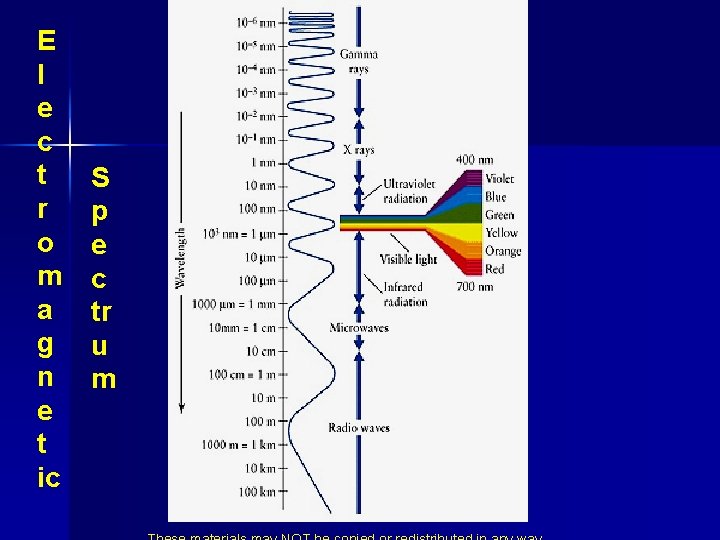
Kinds of EM waves n n There are many kinds of electromagnetic waves different l and n – Radio waves, microwaves, x rays and gamma rays are all examples. n Visible Light is only the part our eyes can detect. Visible Light Bad Gamma Rays High frequency – Low wavelength Radio Waves Good Low frequency – High wavelength

* c = The speed of light n n in a vacuum the speed of light is 2. 998 x 108 m/s The relationship between frequency and wavelength is c = ln

* What is the wavelength of light with a frequency 5. 89 x 105 Hz? c = ln n 2. 998 x 108 m/s = l 5. 89 x 105 Hz n 509 m = l n

* Your Turn What is the frequency of blue light with a wavelength of 484 nm? Ans:

Answer c = ln n 2. 998 x 108 m/s = 484 nm 1 m x n 1 109 nm 2. 998 x 108 m/s = 4. 84 x 10 -7 n n n = 6. 19 x 1014 Hz n

Einstein & Planck Photoelectric Effect and Photons Provides evidence of the particle nature of light. n Also provides evidence for quantization. n The energy of 1 photon = h n

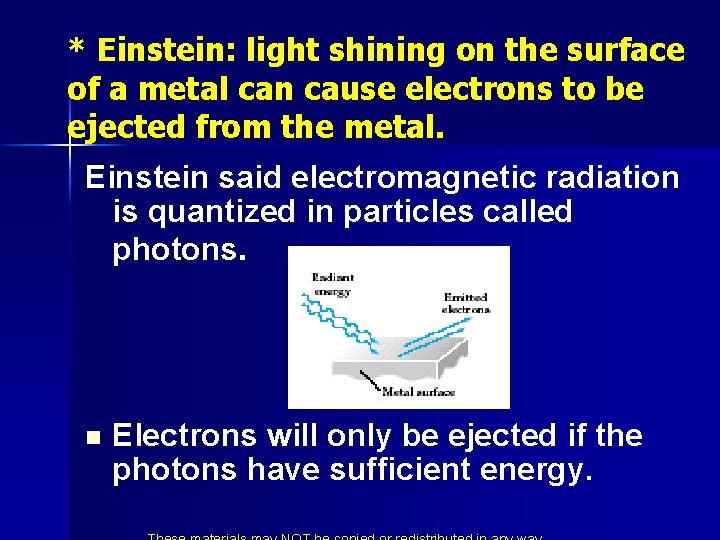
* Einstein: light shining on the surface of a metal can cause electrons to be ejected from the metal. Einstein said electromagnetic radiation is quantized in particles called photons. n Electrons will only be ejected if the photons have sufficient energy.

Photoelectric Effect Video n http: //wps. prenhall. com/wps/media /access/Pearson_Default/3064/3137 997/login. html

* Energy is Quantized Planck found ∆E for a photon came in chunks called quantum. n DEphoton = hn n Øh is Planck’s constant § (h = 6. 626 x 10 -34 J s) Øn is frequency

So is it a Wave or a particle? Is it like light or matter? n n Is energy a wave like light, or a particle like matter? Yes says Einstein! It’s both. Concept is called the Wave - Particle duality. n n What about the other way, is matter a wave? Yes

Diffraction n n When light passes through, or reflects off, a series of thinly spaced line, it creates a rainbow effect because the waves interfere with each other.

Spectrum The range of frequencies present in light. n White light has a continuous spectrum. n All the colors are possible. n A rainbow. n

White Light Continuous Spectrum

E l e c t r o m a g n e t ic S p e c tr u m

Hydrogen Bright Line spectrum n n Called an Emission spectrum because it gives off or emits colors of light. There are just a few discrete lines. 656 nm 434 nm 410 nm 486 nm

What does all this mean? Only certain energies are allowed for the hydrogen atom. n Can only give off certain energies. n Use DE = hn and c= ln so DE = hc / l n Energy in the atom is quantized!! n

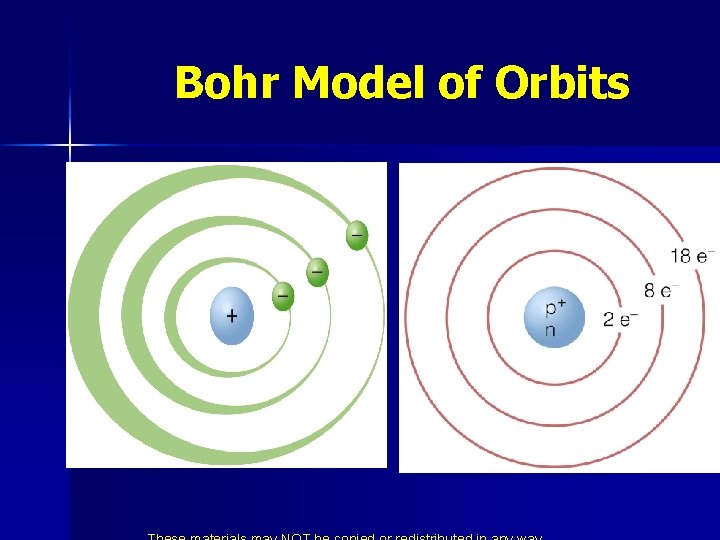
* Niels Bohr n n Developed the quantum model of the hydrogen atom. He said the atom was like a solar system. The electrons were attracted to the nucleus because of opposite charges. Didn’t fall into the nucleus because it is moving around.

Bohr Model of Orbits

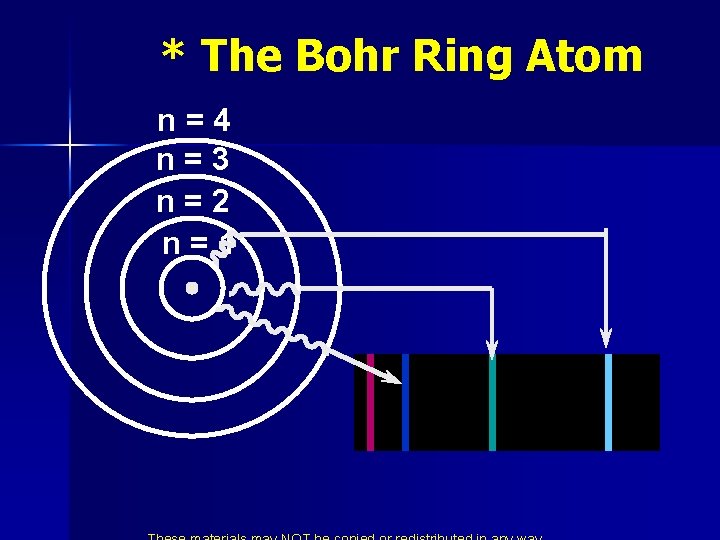
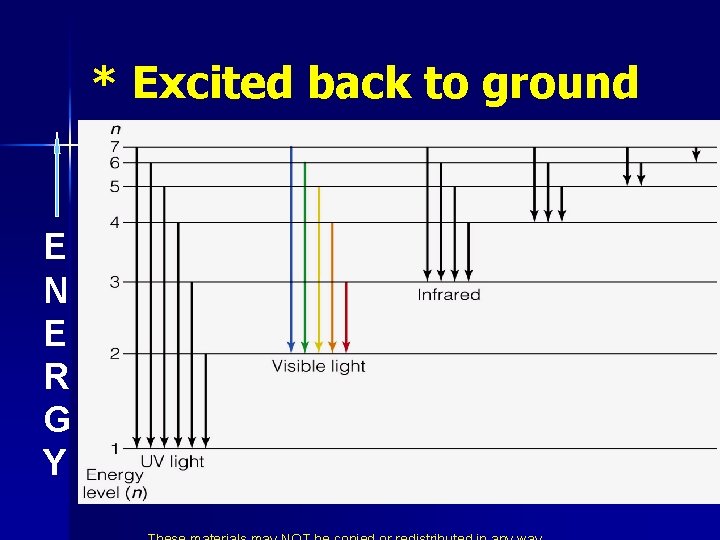
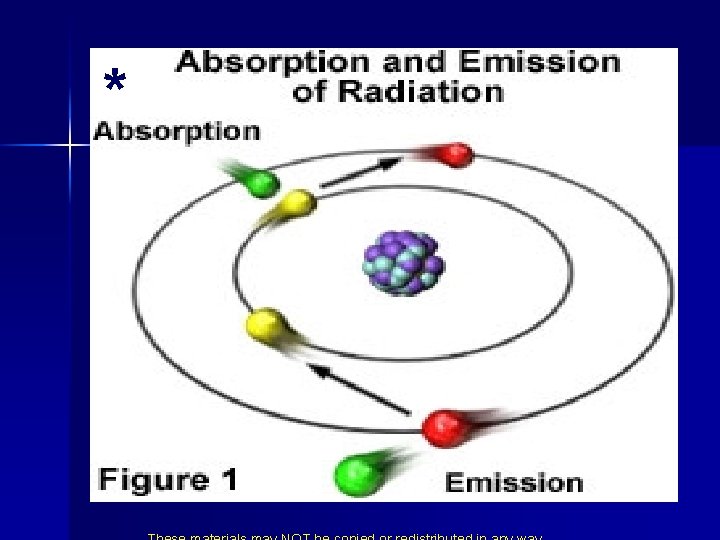
* The Bohr Ring Atom n n n He didn’t know why but only certain energies were allowed. He called these allowed energies energy levels. Putting Energy into the atom moved the electron away from the nucleus. From ground state to excited state. When it returns to ground state it gives off light of a certain energy.

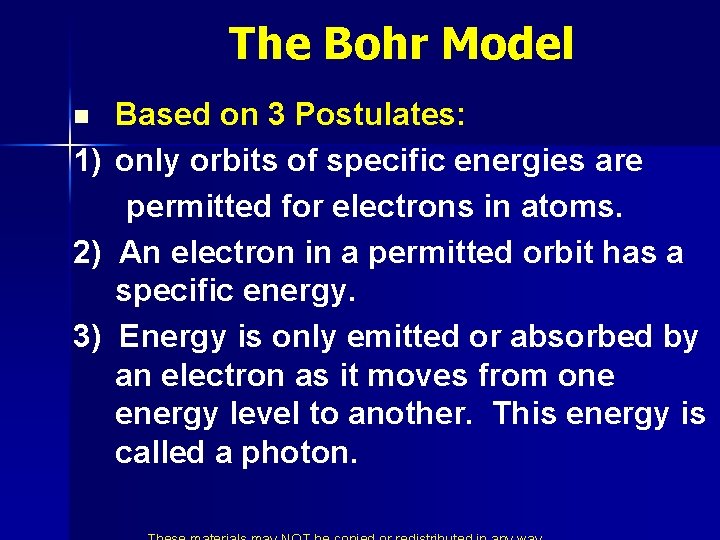
The Bohr Model Based on 3 Postulates: 1) only orbits of specific energies are permitted for electrons in atoms. 2) An electron in a permitted orbit has a specific energy. 3) Energy is only emitted or absorbed by an electron as it moves from one energy level to another. This energy is called a photon. n

Energy States of the Hydrogen Atom n Colors from excited atoms arise as electrons move from the excited state back to the ground state. n Since energy is quantized, the light emitted is quantized bright line spectrum.

* The Bohr Ring Atom n=4 n=3 n=2 n=1

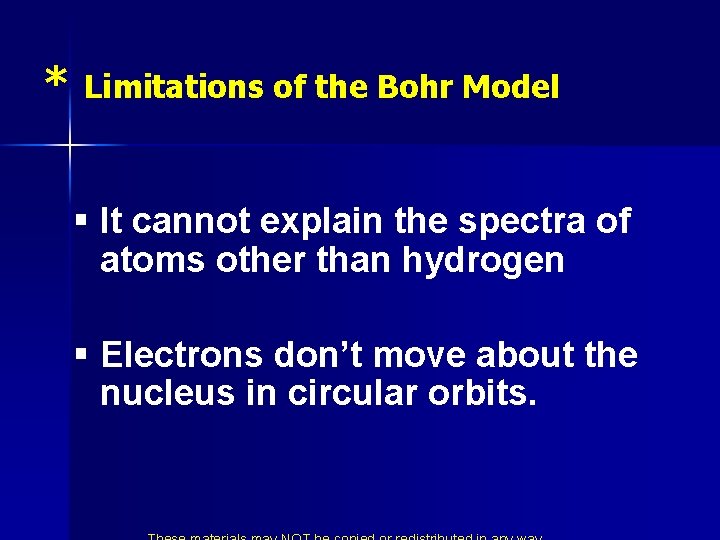
* Limitations of the Bohr Model § It cannot explain the spectra of atoms other than hydrogen § Electrons don’t move about the nucleus in circular orbits.

* What’s good about it? Energy is quantized n Electrons only exist in certain energy levels described by quantum numbers. n Energy gain or loss is involved in moving an electron from one energy level to another. n

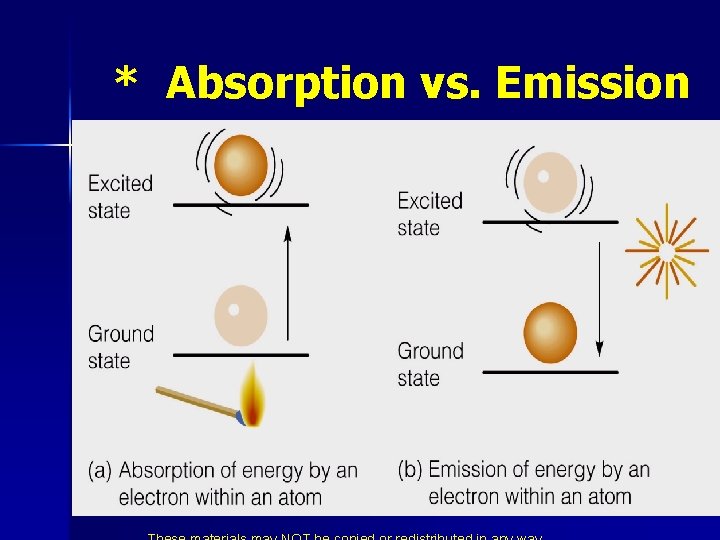
* Schrodinger’s Atomic Model In order for electrons to jump to a higher energy level they must absorb a quantity of energy exactly equal to the energy difference. When electrons jump from a higher to a lower energy level, they give off radiation exactly equal to the energy difference.

* Absorption vs. Emission

* Excited back to ground E N E R G Y


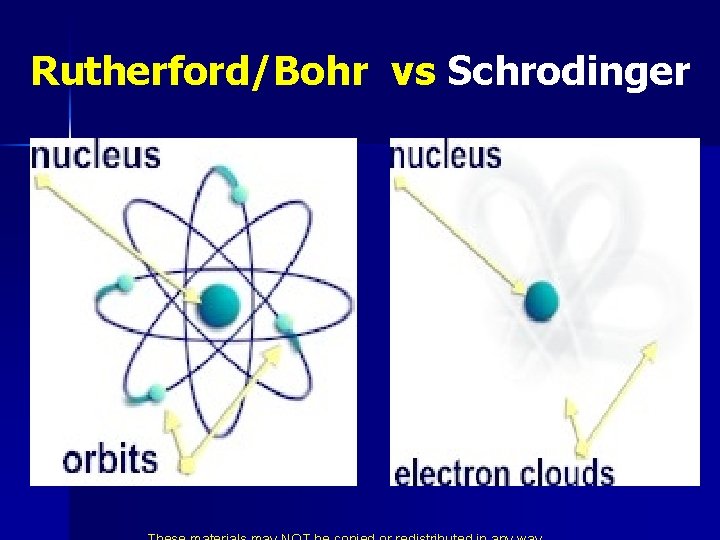
*Schrodinger Killed Bohr’s Atom

* No more orbits they are now orbitals ! Schrodinger’s solution to the equations are called orbitals. n These are not Bohr orbits. n Each solution is tied to a certain energy. n These are the energy levels. n

There is a limit to what we can know: * Heisenberg Uncertainty Principle n We cannot determine the exact position, direction of motion, and speed of subatomic particles simultaneously. FOR ELECTRONS: n We cannot determine their velocity and position simultaneously.

* In other words………. n We can’t know how the electron is moving or how it gets from one energy level to another.

Rutherford/Bohr vs Schrodinger

Today’s Atom

Quantum Numbers n n The numbers that specify the properties of atomic orbitals and of their electrons. There are four numbers needed to describe the electron.

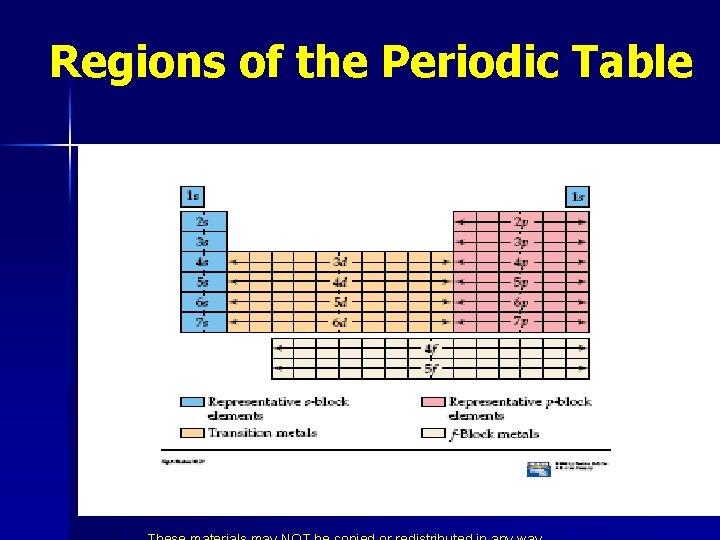
* The four numbers describe: 1) 2) 3) 4) The energy level (1, 2, 3, 4, 5, 6, 7) Also the period on the periodic table. Called the Principle Quantum Number. The shape (s, p, d, f) Also the block on the periodic table. Called the Sublevel or the Orbital Quantum Number. The orbital (s-1. p-3, d-5, f-7). Also called the Magnetic Quantum Number. The spin (up or down arrows). Also called the Spin Quantum Number.

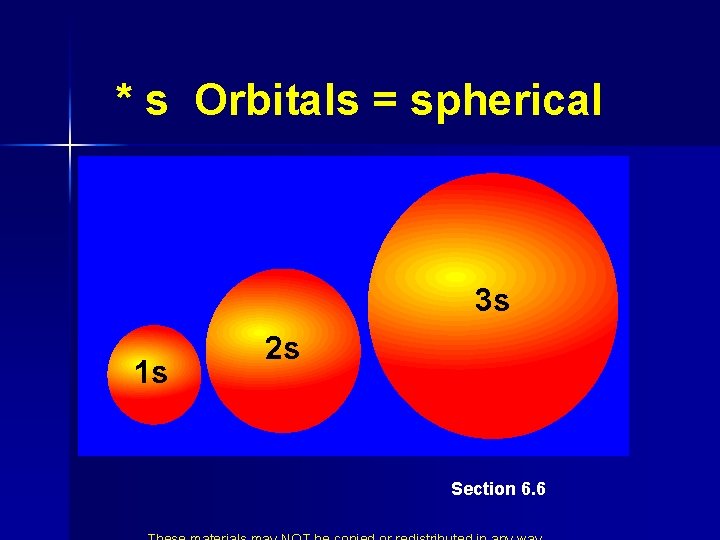
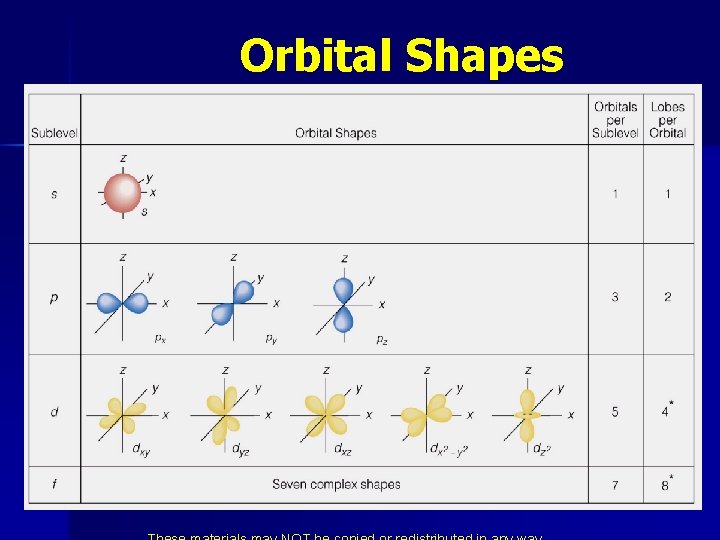
* s Orbitals = spherical 3 s 1 s 2 s Section 6. 6

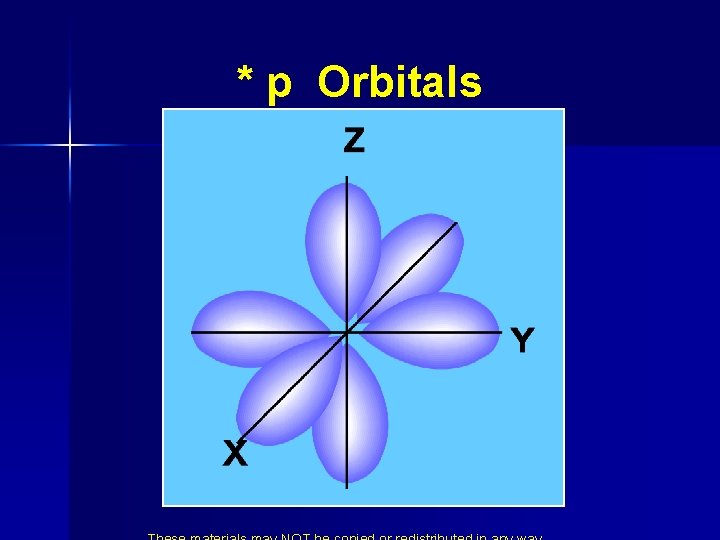
* p Orbitals 2 px 2 py 2 pz

* p Orbitals

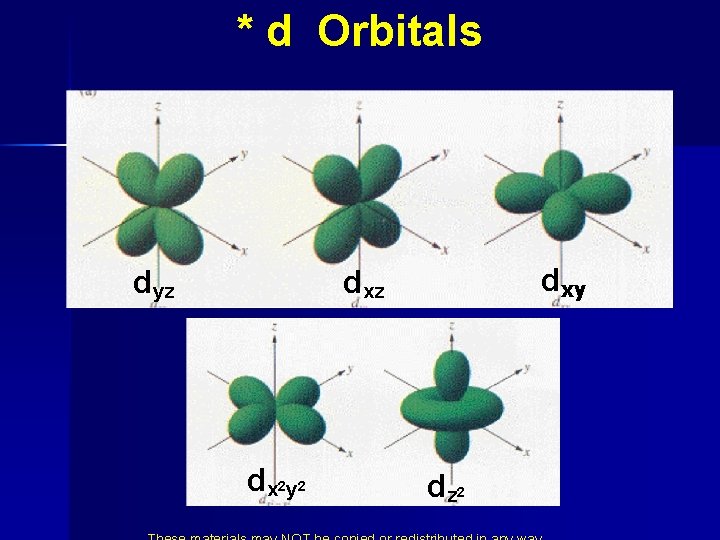
* d Orbitals dyz dxy dxz d x 2 y 2 dz 2

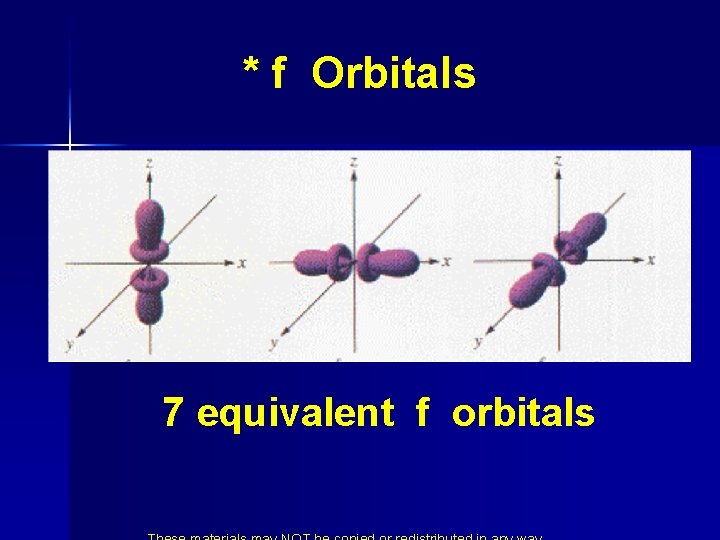
* f Orbitals 7 equivalent f orbitals

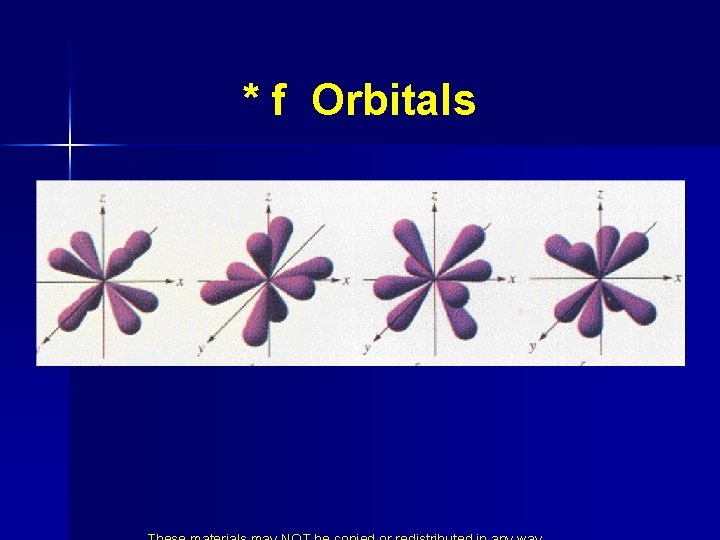
* f Orbitals

Orbital Shapes

* Rules Governing Electron Configuration n n Aufbau Principle Pauli Exclusion Principle Hund’s Rule http: //video. google. com/videoplay? d ocid=-1119064594353208617

Energy Levels Aufbau Principle: • Aufbau is German for building up. • As the protons are added one by one, the electrons fill up hydrogenlike orbitals. • Fill up in order of energy levels.

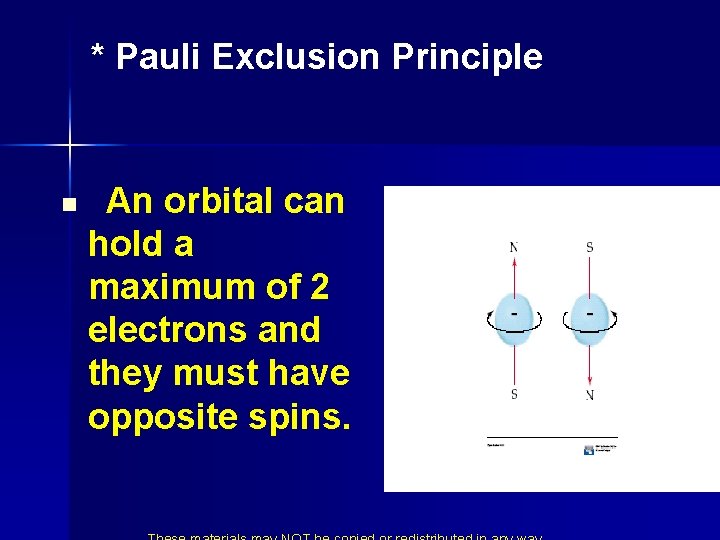
* Pauli Exclusion Principle Ø NO TWO ELECTRONS IN AN ATOM CAN HAVE THE SAME SET OF FOUR QUANTUM NUMBERS Therefore…………. .

* Pauli Exclusion Principle n An orbital can hold a maximum of 2 electrons and they must have opposite spins.

* Hund’s Rule n Orbitals of equal energy are each occupied by one electron before any one orbital is occupied by a second electron, and all electrons in singly occupied orbitals must have the same spin.

Filling Order n http: //video. google. com/videoplay? docid= 5436250208251878519&q=electron+confi guration&total=28&start=0&num=10&so =0&type=search&plindex=0 n http: //intro. chem. okstate. edu/Workshop. F older/Electronconfnew. html

Electron Configurations – The way electrons are distributed among the various orbitals.

Formulas to determine number of orbitals and electrons Number of Orbitals = 2 n Number of Electrons = 2 2 n

E n e r g y


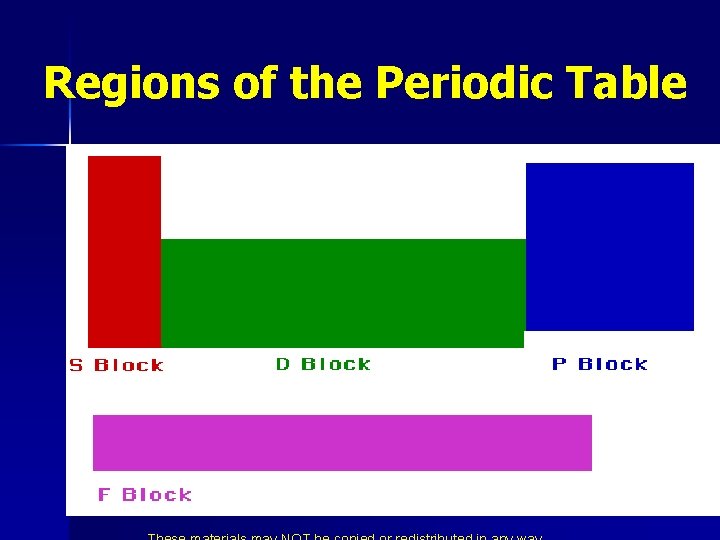
Regions of the Periodic Table

Regions of the Periodic Table

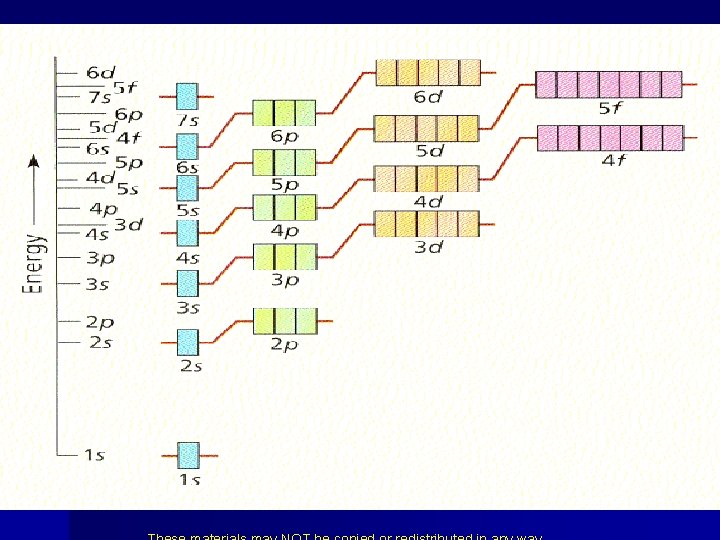
Let’s learn electron configuration from the periodic Table.

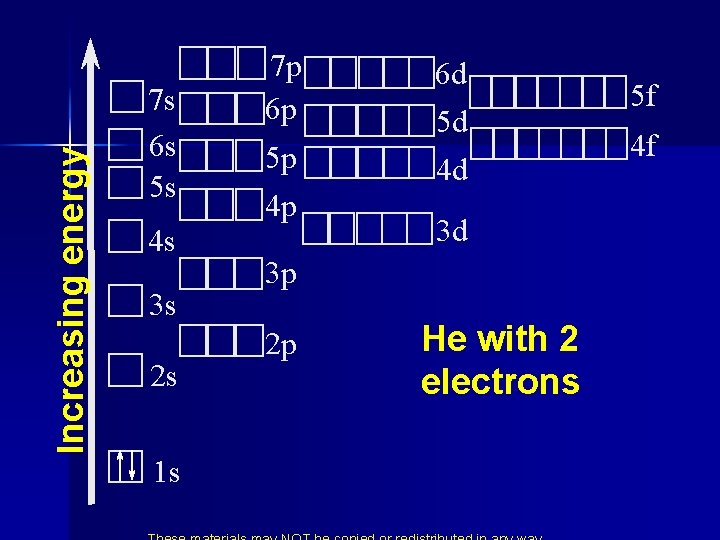
Increasing energy 7 s 6 s 5 s 4 s 3 s 2 s 1 s 7 p 6 p 5 p 4 p 6 d 5 d 4 d 3 d 3 p 2 p He with 2 electrons 5 f 4 f

Details n n n Valence electrons- the electrons in the outermost energy levels (not d or f). Core electrons- the inner electrons. C 1 s 2 2 p 2

Fill from the bottom up following the arrows 7 s 7 p 7 d 7 f 6 s 6 p 6 d 6 f 5 s 5 p 5 d 5 f 4 s 4 p 4 d 4 f 3 s 3 p 3 d 2 s 2 p 1 s • 2 1 s 2 2 s 6 2 p 2 3 s 6 3 p 2 4 s 10 3 d 6 4 p 2 5 s 10 6 4 d 5 p 56 • 38 20 electrons 4212 2 6 s

n n n Elements in the same column have the same electron configuration. Put in columns because of similar properties. Similar properties because of electron configuration. Noble gases have filled energy levels. Transition metals are filling the d orbitals

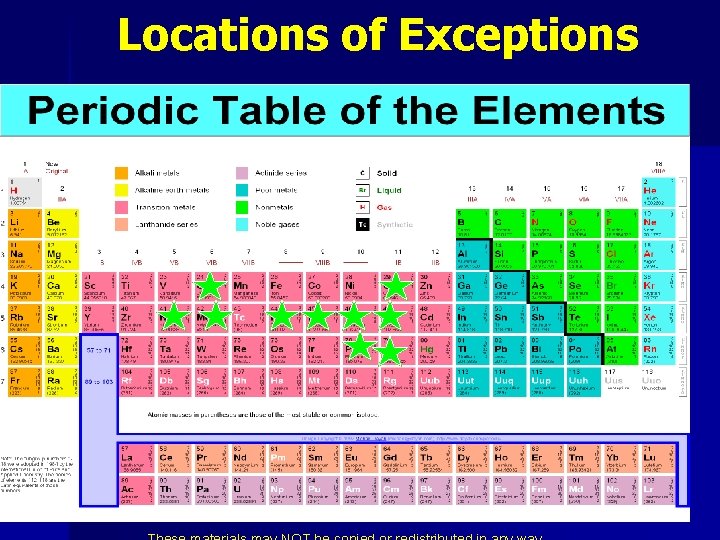
Exceptions ØHalf filled s orbital ØScientists aren’t sure of why it happens n n Cr = [Ar] 4 s 1 3 d 5 Cu = [Ar] 4 s 1 3 d 10 Nb = [Kr] 5 s 1 4 d 4 Mo = [Kr] 5 s 1 4 d 5 Ru = [Kr] 5 s 1 4 d 7 Rh = [Kr] 5 s 1 4 d 8 Pd = [Kr] 4 d 10 Ag = [Kr] 5 s 1 4 d 10 Pt = [Xe] 6 s 1 5 d 10 Au = [Xe] 6 s 1 4 f 14 5 d 10

Locations of Exceptions