Chapter 4 Compounds and Their Bonds Octet Rule

Chapter 4 �Compounds and Their Bonds � Octet Rule and Ions � Ionic Compounds � Naming and Writing Ionic Formulas � Polyatomic Ions � Covalent Compounds � Electronegativity and Bond Polarity � Shapes and Polarity of Molecules � Attractive Forces in Compounds 12/19/2021 1
Evidence for the Octet Rule �Each filled shell (group 8 A, noble gases) has eight valence electrons. �Noble gases tend not to react. �Stability of atom due to these eight electrons. �Atoms like sodium tend to always lose one electron. �Atoms like chlorine tend to always gain one electron. 12/19/2021 2
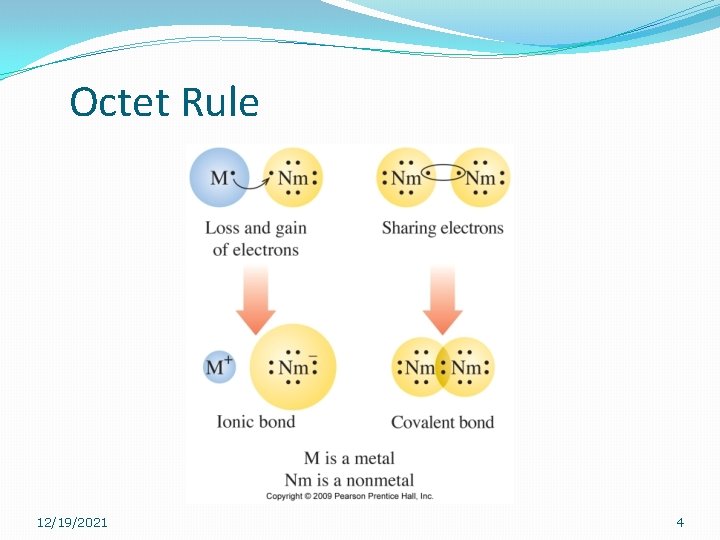
Octet Rule �Atoms in a compound will lose, gain, or share eight electrons. �Two types of compounds: Ionic and Covalent �Ionic – atoms gain or lose electrons to form ions. �Covalent – atoms share electrons 12/19/2021 3
Octet Rule 12/19/2021 4

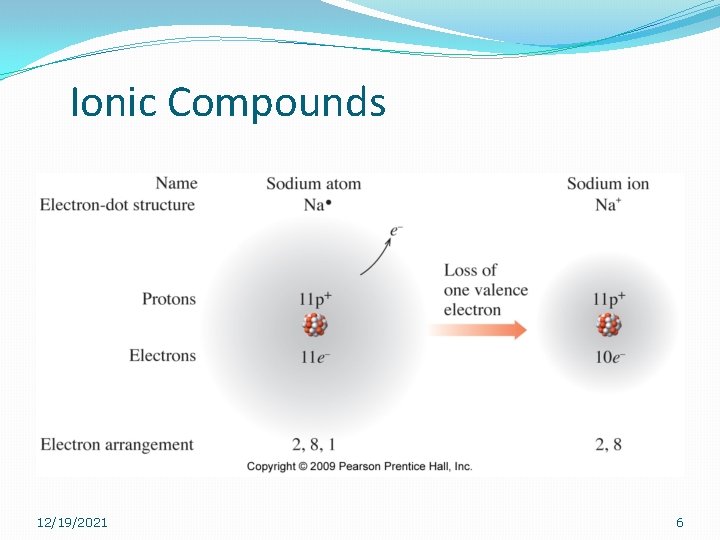
Ionic Compounds �Metals will always lose electrons. �Group 1 A metals will always lose one electron. �What is its charge (valence)? �Group 2 A metals will always lose two electrons. �What is its valence? 12/19/2021 5
Ionic Compounds 12/19/2021 6
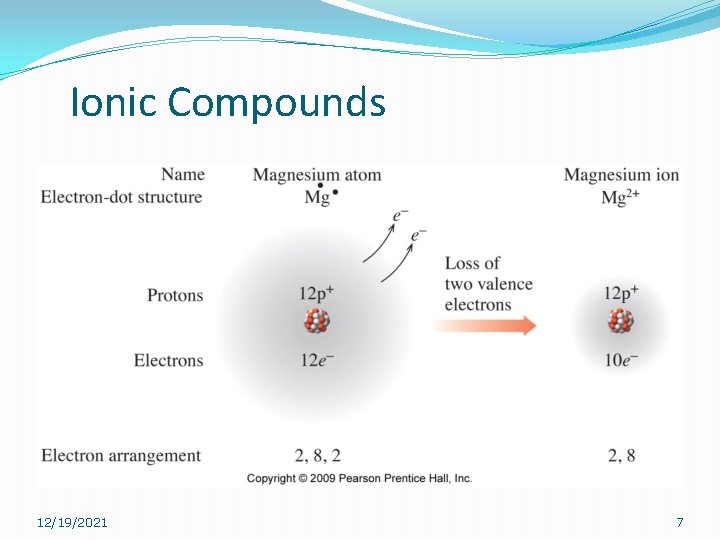
Ionic Compounds 12/19/2021 7

Ionic Compounds �Non-metals (in an ionic compound) will always gain electrons. �Group 7 A will always gain one electron. �What is the valence of this group? �Group 6 A (Oxygen and Sulfur) will always gain two electrons �What are their valences? 12/19/2021 8
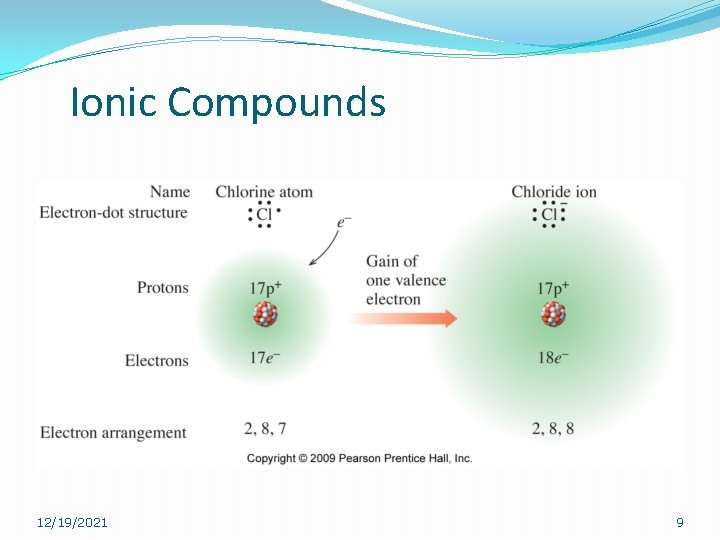
Ionic Compounds 12/19/2021 9

Learning Check �Predicting charges… �What valence will Barium have? �What valence will Bromine have? �What valence will Aluminum have? �What valence will Nitrogen have? 12/19/2021 10
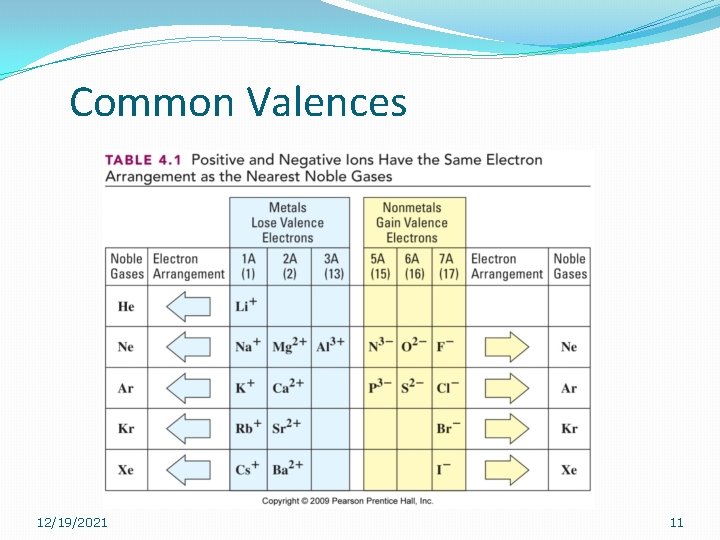
Common Valences 12/19/2021 11
Learning Check �Potassium (K) in a compound will �a) gain one electron �b) lose one electron �Potassium will have a valence of �a) – 1 �b) +1 12/19/2021 12
Learning Check �Sulfur (S) in an ionic compound will �a) gain two electrons �b) lose two electrons �Sulfur will have a valence of �a) – 2 �b) +2 12/19/2021 13

Ionic Compounds �All ionic compounds consist of both positive ions (cations) and negative ions (anions). �Why both? �Ionic compounds are always solids at room temperature with high melting points. �MP of Na. Cl = 801 o. C 12/19/2021 14

Ionic Compounds �An ionic formula is �always written with the cation first followed by the anion. �always electrically neutral – that means the total positive charges must equal the total negative charges. �always empirical – lowest whole number subscripts. 12/19/2021 15

Ionic Compounds �A subscript of “ 1” is always understood and never written. �ex) Na. Cl, consists of one sodium cation and one chlorine anion. �Subscripts of 2, 3, 4, etc. are added after each symbol when needed. �ex) Al 2 O 3 consists of two aluminum cations and three oxygen anions. 12/19/2021 16
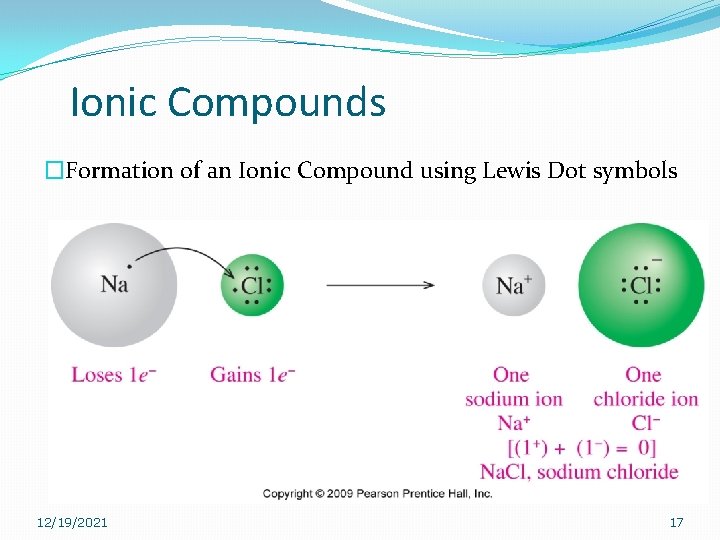
Ionic Compounds �Formation of an Ionic Compound using Lewis Dot symbols 12/19/2021 17
Learning Check �Show the formation of Ca. F 2 and Li 2 S using Lewis Dot symbols. 12/19/2021 18
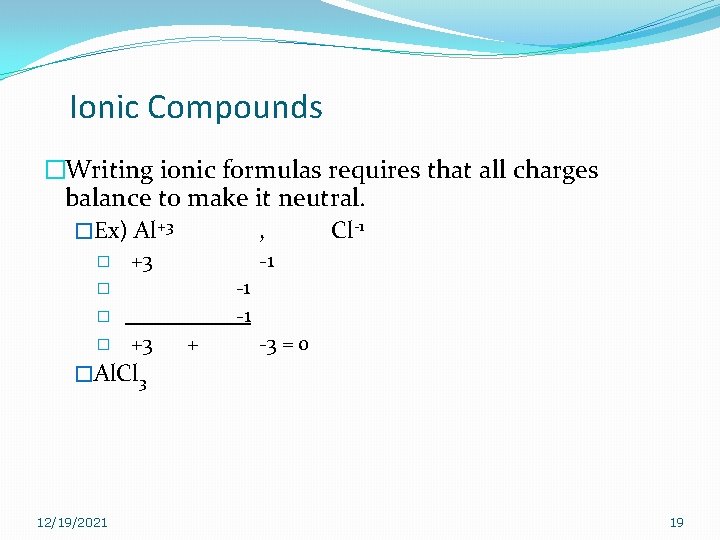
Ionic Compounds �Writing ionic formulas requires that all charges balance to make it neutral. �Ex) Al+3 , Cl-1 � +3 -1 -1 -1 � � � +3 + -3 = 0 �Al. Cl 3 12/19/2021 19
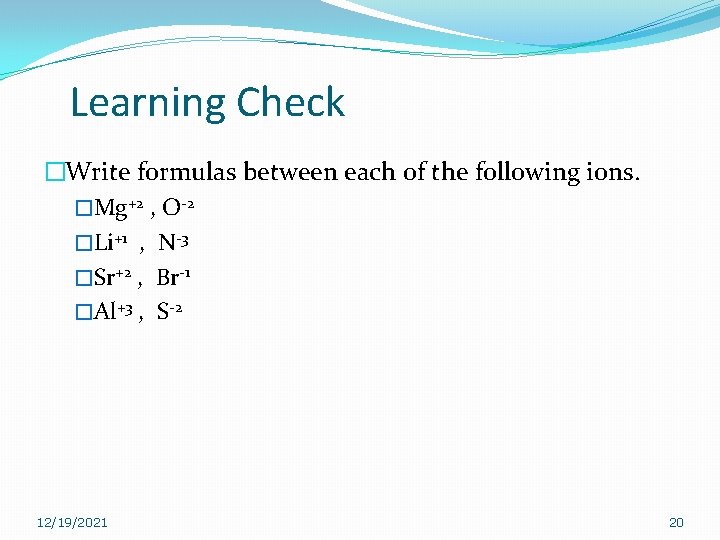
Learning Check �Write formulas between each of the following ions. �Mg+2 , O-2 �Li+1 , N-3 �Sr+2 , Br-1 �Al+3 , S-2 12/19/2021 20

Nomenclature �Naming ionic compounds requires that you determine whether the cation (metal) has a fixed or a variable charge. �Main group metals have fixed charges. �Exception: Sn and Pb 12/19/2021 21

Nomenclature � Transition group metals have variable charges. � Exception: Ag and Zn � Naming an ionic compound whose cation has a fixed charge: 1. Name the metal (cation) first. 2. Name the non-metal (anion) second, but change its suffix to –ide. 12/19/2021 22

Nomenclature � Naming an ionic compound whose cation has a variable charge: 1. Determine the charge of the cation by deduction. 2. Name the metal (cation) first followed by its charge in (roman numerals). 3. Name the non-metal (anion) second, but change its suffix to –ide. 12/19/2021 23
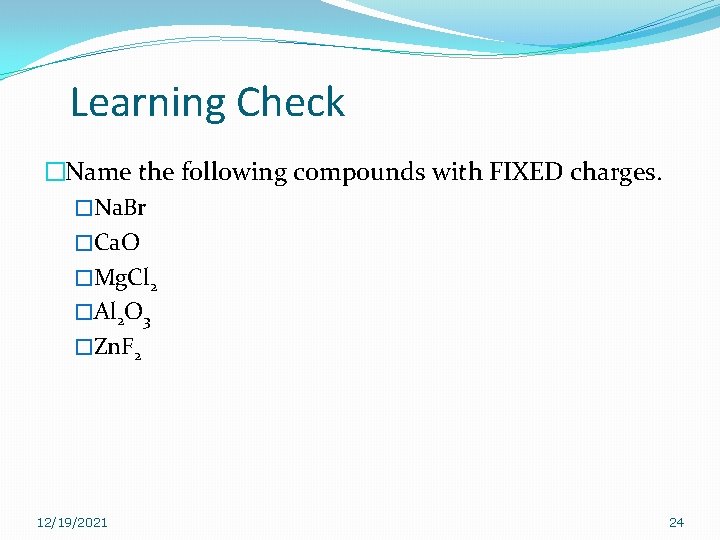
Learning Check �Name the following compounds with FIXED charges. �Na. Br �Ca. O �Mg. Cl 2 �Al 2 O 3 �Zn. F 2 12/19/2021 24
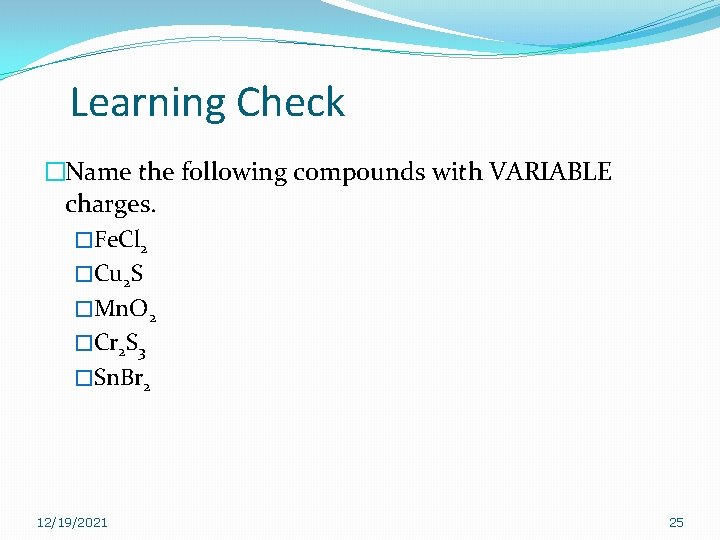
Learning Check �Name the following compounds with VARIABLE charges. �Fe. Cl 2 �Cu 2 S �Mn. O 2 �Cr 2 S 3 �Sn. Br 2 12/19/2021 25
Nomenclature �Writing formulas from a name. �Once again, an ionic compound is electrically neutral. �Must figure out how many of each ion is needed to make it neutral. �Roman numeral (if present) is that metals valence. 12/19/2021 26
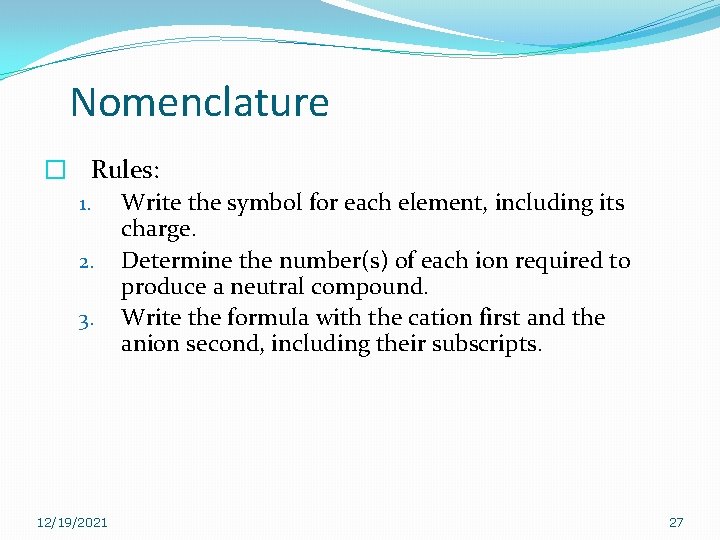
Nomenclature � Rules: 1. Write the symbol for each element, including its charge. 2. Determine the number(s) of each ion required to produce a neutral compound. 3. Write the formula with the cation first and the anion second, including their subscripts. 12/19/2021 27

Learning Check �Write the formulas for: �sodium oxide �aluminum iodide �lead(II) sulfide �iron(III) fluoride �magnesium nitride 12/19/2021 28
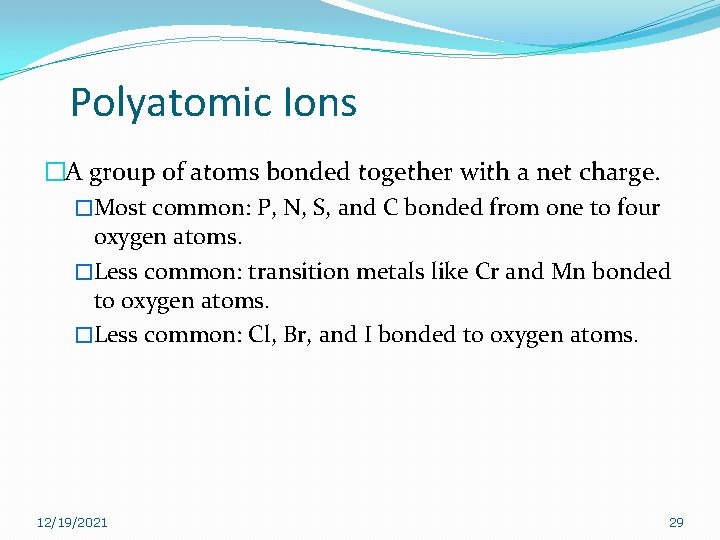
Polyatomic Ions �A group of atoms bonded together with a net charge. �Most common: P, N, S, and C bonded from one to four oxygen atoms. �Less common: transition metals like Cr and Mn bonded to oxygen atoms. �Less common: Cl, Br, and I bonded to oxygen atoms. 12/19/2021 29
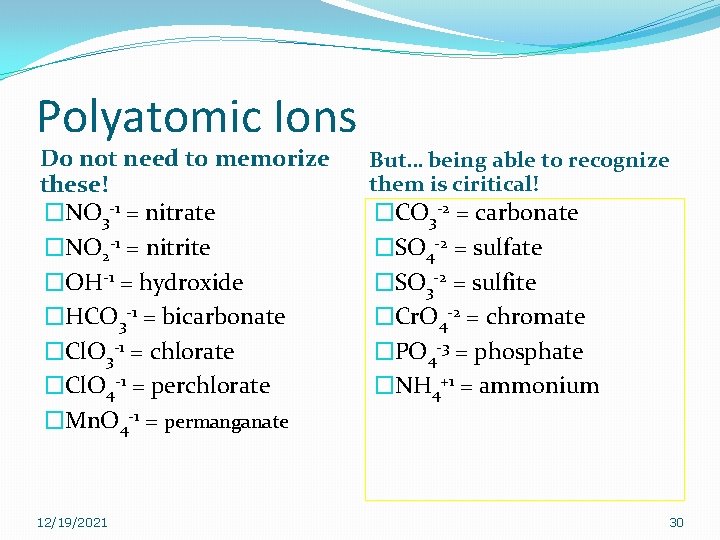
Polyatomic Ions Do not need to memorize these! �NO 3 -1 = nitrate �NO 2 -1 = nitrite �OH-1 = hydroxide �HCO 3 -1 = bicarbonate �Cl. O 3 -1 = chlorate �Cl. O 4 -1 = perchlorate �Mn. O 4 -1 = permanganate 12/19/2021 But… being able to recognize them is ciritical! �CO 3 -2 = carbonate �SO 4 -2 = sulfate �SO 3 -2 = sulfite �Cr. O 4 -2 = chromate �PO 4 -3 = phosphate �NH 4+1 = ammonium 30

Polyatomic Ions �These ions ALWAYS keep there name. �Changing the name alters the meaning! �Ex) Na 2 SO 4 = sodium sulfate �If you called this “sodium sulfide”, then the formula is: Na 2 S. 12/19/2021 31

Naming Compounds with polyatomic ions � Note: if the ammonium ion is present, then name it first followed by the name of the anion with the –ide suffix. 1. Identify the metal present as having a fixed or variable charge. If fixed, name the metal. b) If variable, name the metal followed by its charge in roman numerals. a) 12/19/2021 32

Naming Compounds with polyatomic ions 2. Name the polyatomic ion present – keeping its name EXACTLY the same as on the handout. � Ex) K 2 CO 3 = � Ex) Fe. SO 4 = 12/19/2021 33

Writing Formulas �Once again, all ionic formulas must have an equal number of positive and negative charges. �If more than one polyatomic ion is required, then it is put in parenthesis with a subscript outside of these. 12/19/2021 34
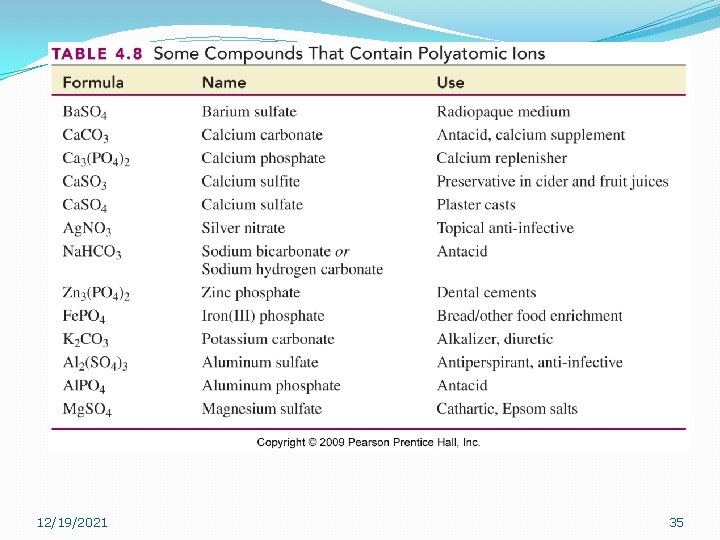
12/19/2021 35
Learning Check �Name each compound. �Na 3 PO 4 �Zn(OH)2 = �V(NO 3)3 = 12/19/2021 36

Learning Check �Write the formulas for: �lithium nitrite �nickel(II) chlorate �aluminum sulfate 12/19/2021 37
Covalent Compounds �Covalent bonds form when two atoms share electrons to achieve an octet. �Simplest case is when two Hydrogen atoms form an H 2 molecule. �Each atom has one electron. �Note that an “octet” for Hydrogen is two electrons. 12/19/2021 38
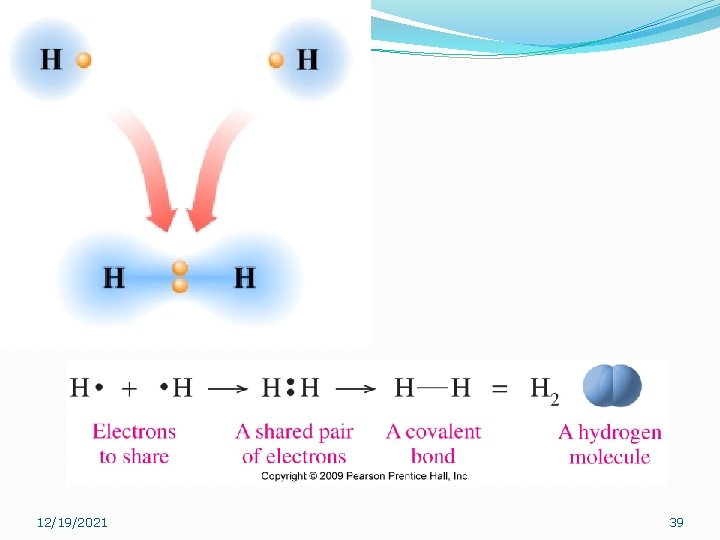
12/19/2021 39
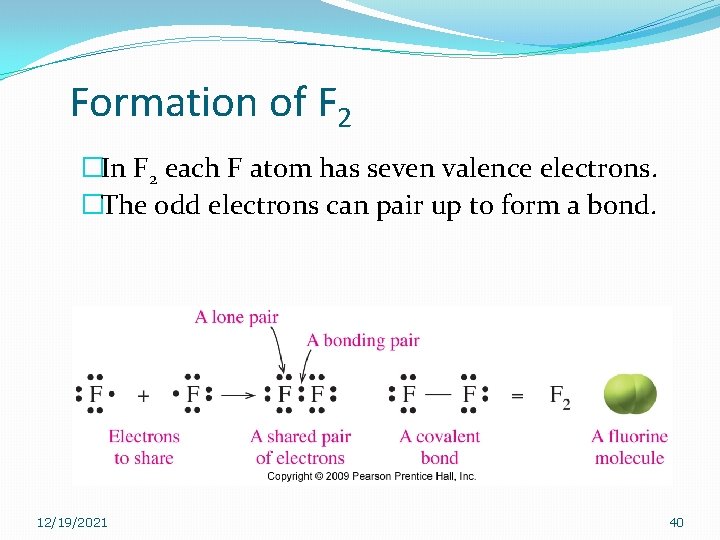
Formation of F 2 �In F 2 each F atom has seven valence electrons. �The odd electrons can pair up to form a bond. 12/19/2021 40
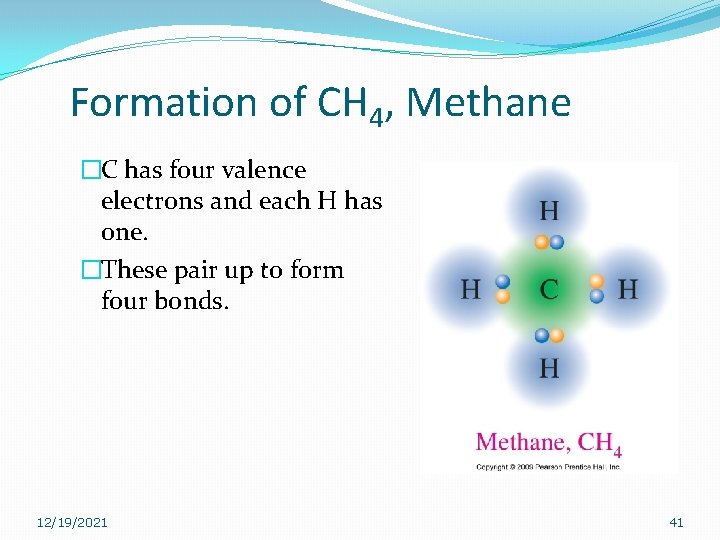
Formation of CH 4, Methane �C has four valence electrons and each H has one. �These pair up to form four bonds. 12/19/2021 41
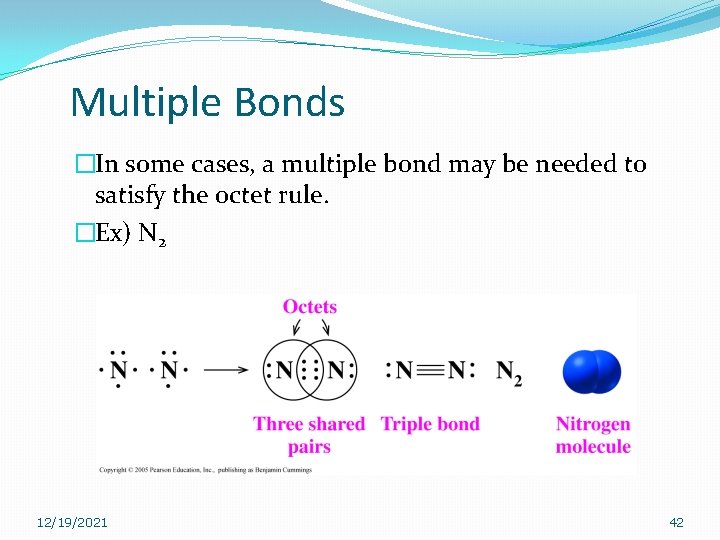
Multiple Bonds �In some cases, a multiple bond may be needed to satisfy the octet rule. �Ex) N 2 12/19/2021 42

Making a Lewis Structure � In general, a Lewis Structure can be set up as follows. 1. Determine the total number of valence electrons from all atoms in the formula. 2. Set up a skeleton structure by putting the first element in formula in the middle. Place all the others around this central atom. 12/19/2021 43
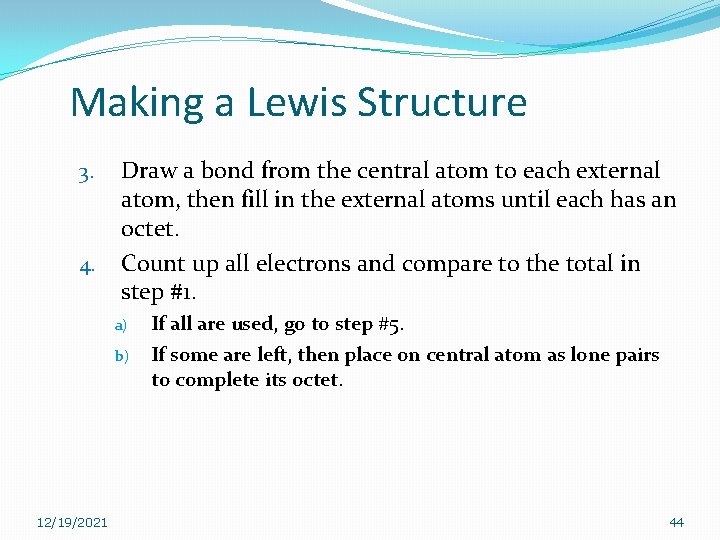
Making a Lewis Structure 3. 4. Draw a bond from the central atom to each external atom, then fill in the external atoms until each has an octet. Count up all electrons and compare to the total in step #1. a) b) 12/19/2021 If all are used, go to step #5. If some are left, then place on central atom as lone pairs to complete its octet. 44
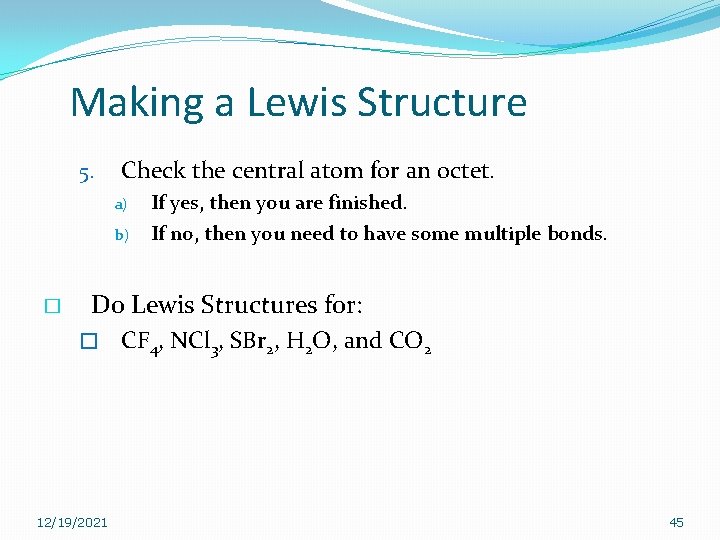
Making a Lewis Structure 5. Check the central atom for an octet. a) b) � If yes, then you are finished. If no, then you need to have some multiple bonds. Do Lewis Structures for: � 12/19/2021 CF 4, NCl 3, SBr 2, H 2 O, and CO 2 45

Naming Covalent Compounds �Covalent compounds use prefixes to indicate the number of atoms of each type. �one = mono (used only for second element!) �two = di �three = tri �four = tetra �five = penta �six = hexa 12/19/2021 46

Naming Covalent Compounds �Name the first element in the formula – add the appropriate prefix if there is more than one. �Name the second element in the formula – change the suffix to –ide – include a prefix to indicate the subscript even if it is “ 1”. 12/19/2021 47

Learning Check �Name: �CO 2 �NO �NCl 3 �PCl 5 12/19/2021 48

Writing a Covalent Formula �Since electrons are shared, charges do NOT apply. �Simply look at the prefixes and apply them. �Ex) sulfur trioxide = �Ex) dinitrogen tetroxide = 12/19/2021 49
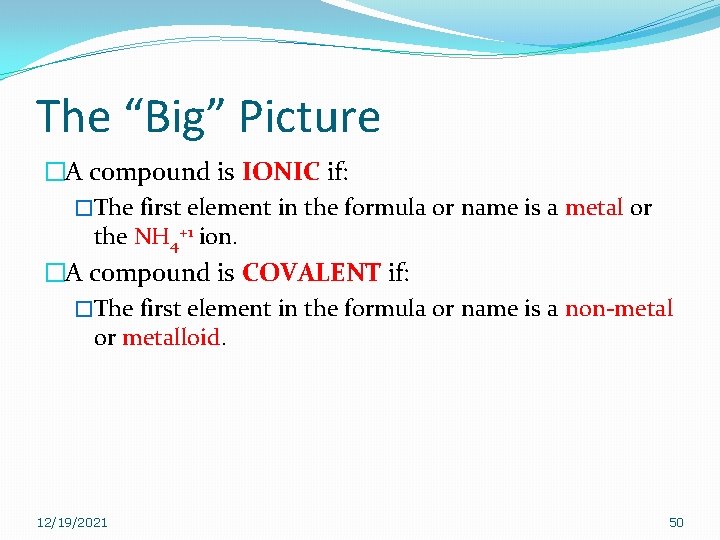
The “Big” Picture �A compound is IONIC if: �The first element in the formula or name is a metal or the NH 4+1 ion. �A compound is COVALENT if: �The first element in the formula or name is a non-metal or metalloid. 12/19/2021 50
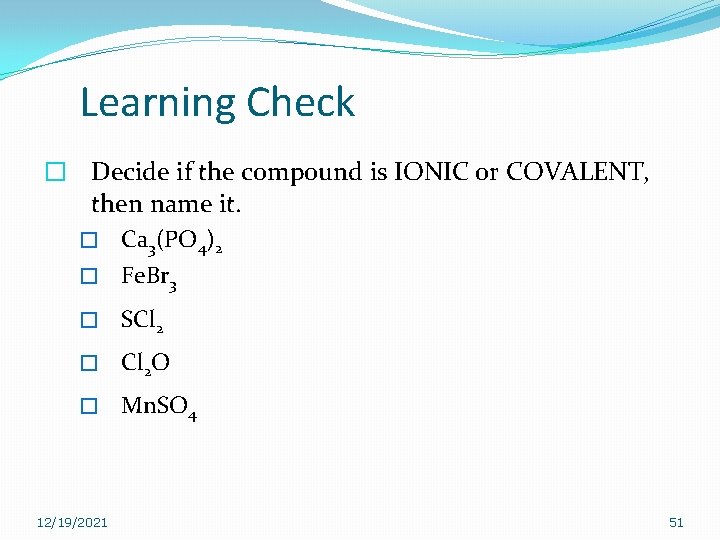
Learning Check � Decide if the compound is IONIC or COVALENT, then name it. � Ca 3(PO 4)2 � Fe. Br 3 � SCl 2 � Cl 2 O � Mn. SO 4 12/19/2021 51
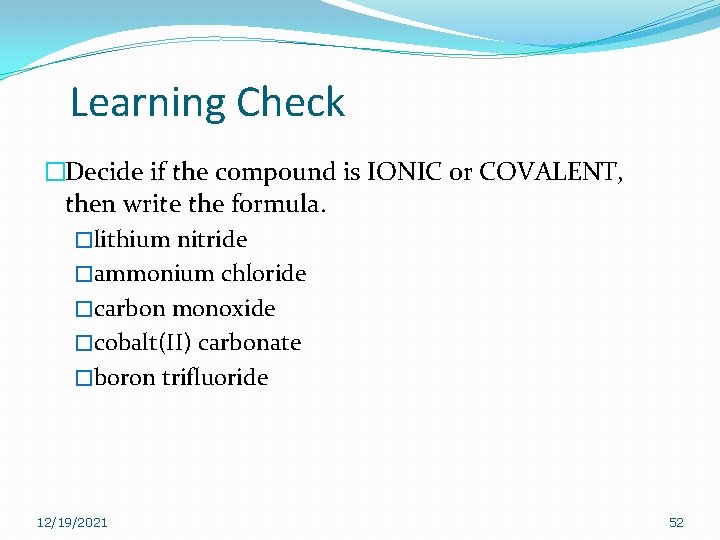
Learning Check �Decide if the compound is IONIC or COVALENT, then write the formula. �lithium nitride �ammonium chloride �carbon monoxide �cobalt(II) carbonate �boron trifluoride 12/19/2021 52
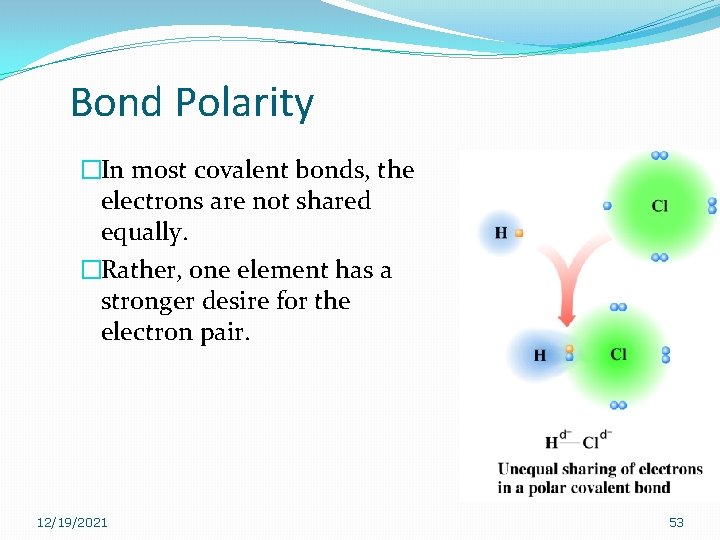
Bond Polarity �In most covalent bonds, the electrons are not shared equally. �Rather, one element has a stronger desire for the electron pair. 12/19/2021 53

Electronegativity �The ability of an atom to attract the electron pair in the bond is called its electronegativity. �The element fluorine has the greatest desire and is assigned the value of 4. 0. �All others are compared to fluorine. 12/19/2021 54
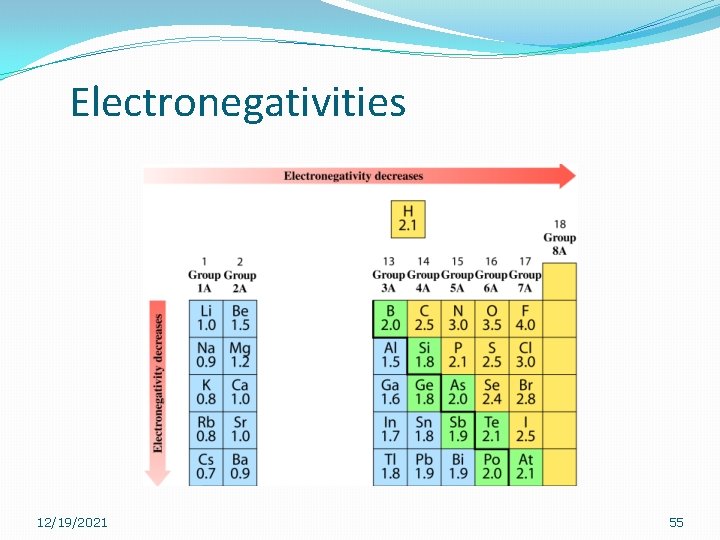
Electronegativities 12/19/2021 55
Covalent Bonds �Non-polar Covalent �Occurs when the difference in electronegativity is zero. �Ex) Cl-Cl, O-O, and N-Cl �Polar Covalent �All other covalent bonds �Ex) H-I, N-F, O-H, H-F 12/19/2021 56

Ionic Bonds �Always occur between metal and non-metal – regardless of difference in electronegativity. �Ex) Na-Cl, Mg-S, Al-Br 12/19/2021 57
Polar Covalent Bonds �Use a partial positive charge (d+) over the less electronegative atom. �Use a partial negative charge (d-) over the more electronegative atom. �Ex) H Cl d+ 12/19/2021 d- 58
Shapes of Molecules �VSEPR theory – valence shell electron pair repulsion �States that bonding pairs of electrons will completely repel each other �We will limit this to two, three, or four pairs of electrons. 12/19/2021 59
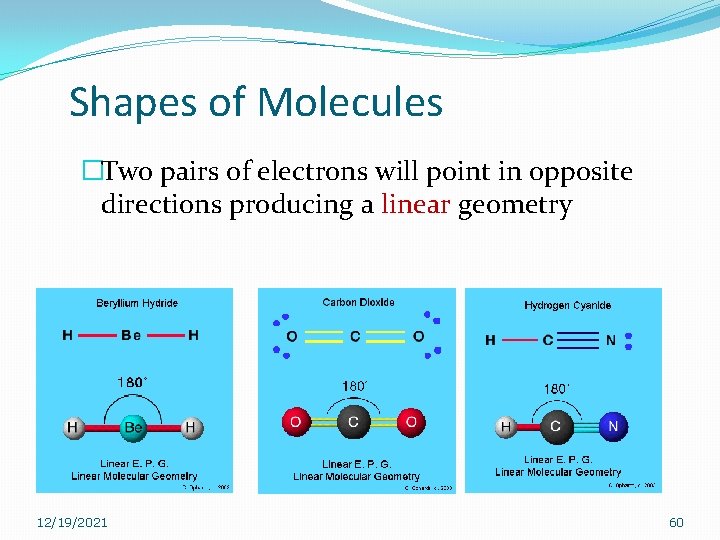
Shapes of Molecules �Two pairs of electrons will point in opposite directions producing a linear geometry 12/19/2021 60
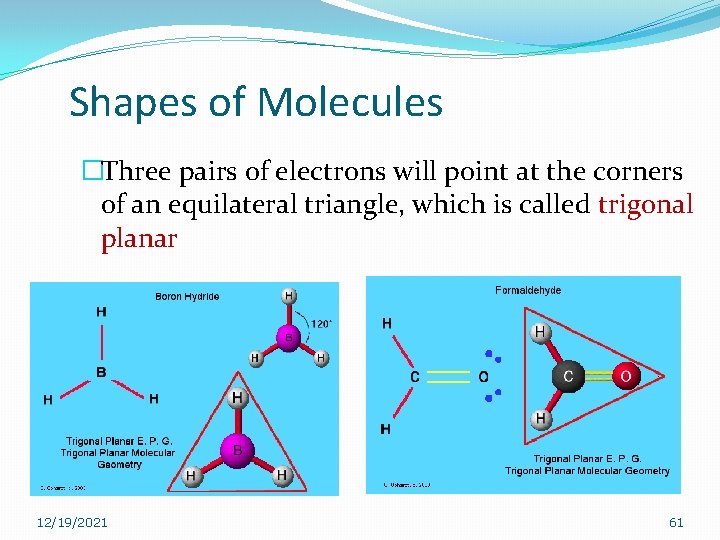
Shapes of Molecules �Three pairs of electrons will point at the corners of an equilateral triangle, which is called trigonal planar 12/19/2021 61
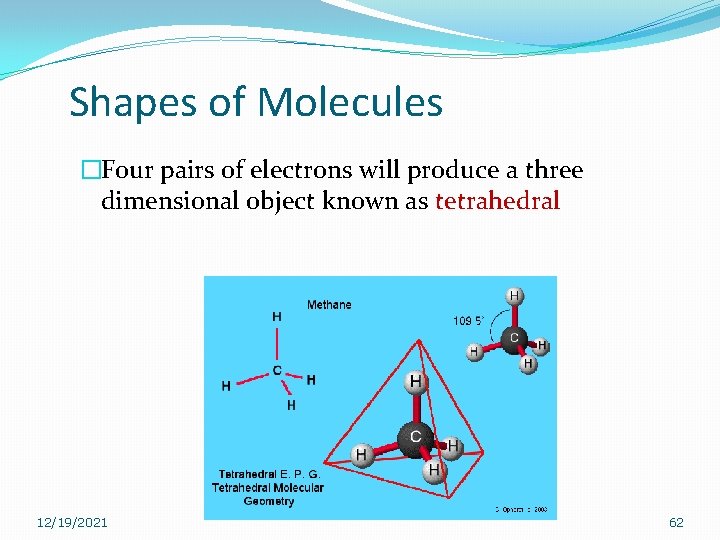
Shapes of Molecules �Four pairs of electrons will produce a three dimensional object known as tetrahedral 12/19/2021 62
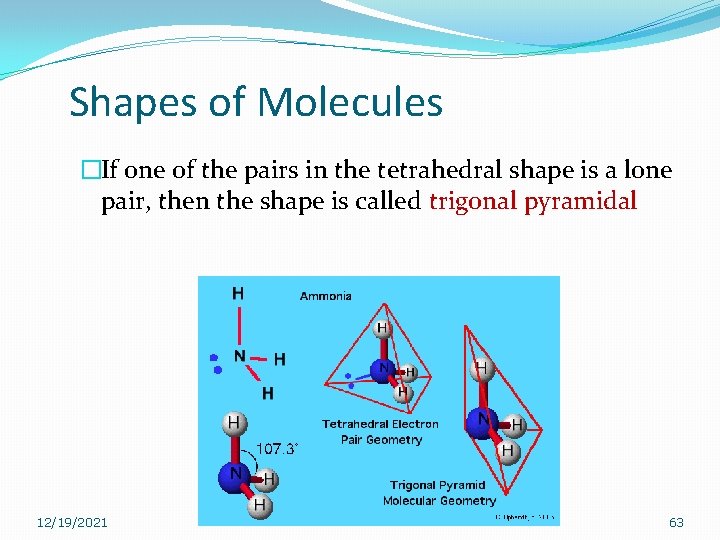
Shapes of Molecules �If one of the pairs in the tetrahedral shape is a lone pair, then the shape is called trigonal pyramidal 12/19/2021 63
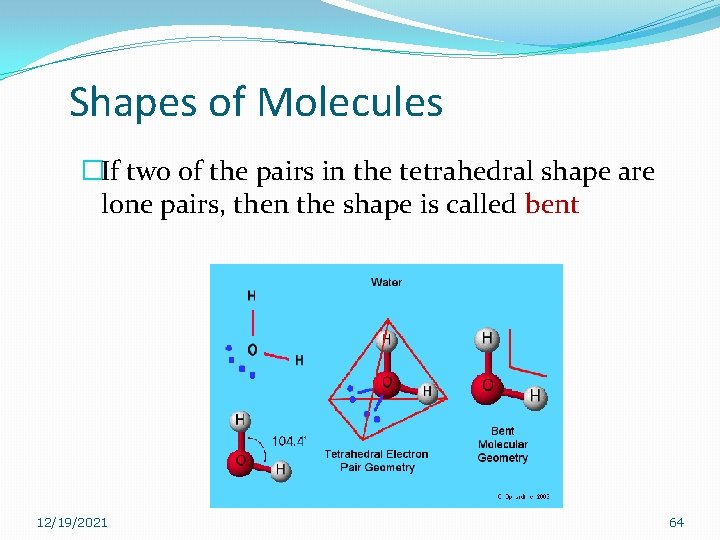
Shapes of Molecules �If two of the pairs in the tetrahedral shape are lone pairs, then the shape is called bent 12/19/2021 64
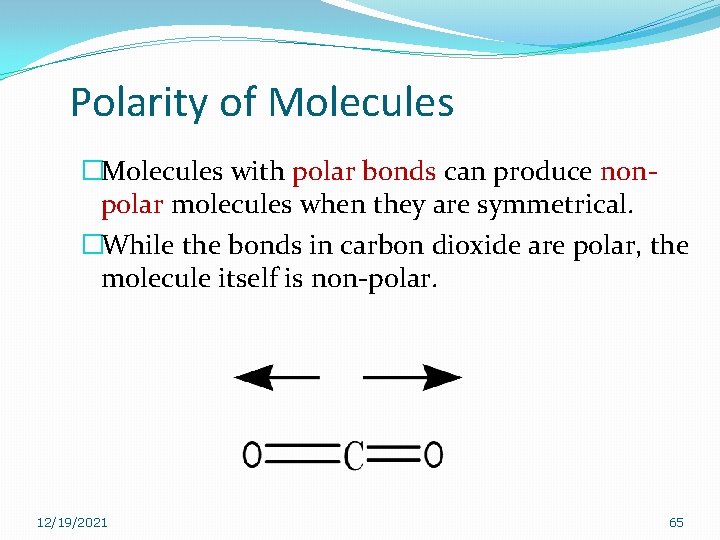
Polarity of Molecules �Molecules with polar bonds can produce nonpolar molecules when they are symmetrical. �While the bonds in carbon dioxide are polar, the molecule itself is non-polar. 12/19/2021 65
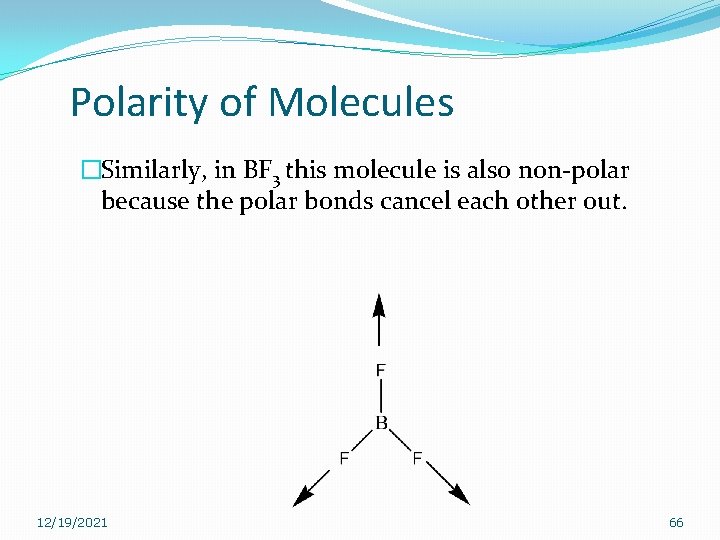
Polarity of Molecules �Similarly, in BF 3 this molecule is also non-polar because the polar bonds cancel each other out. 12/19/2021 66
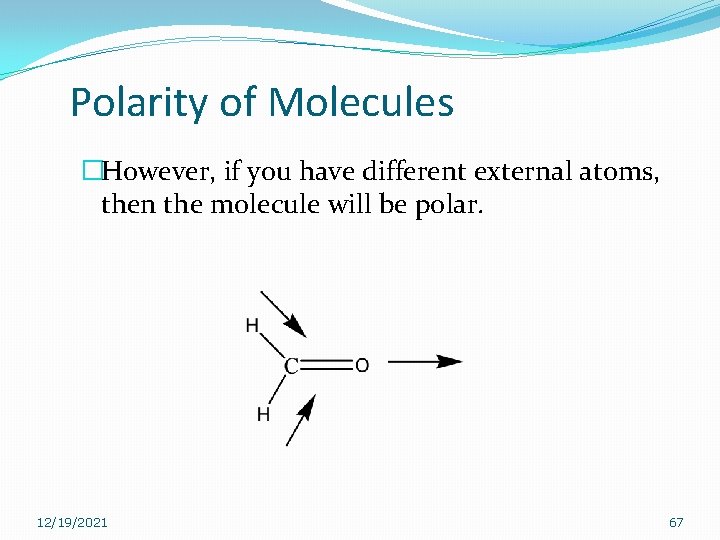
Polarity of Molecules �However, if you have different external atoms, then the molecule will be polar. 12/19/2021 67
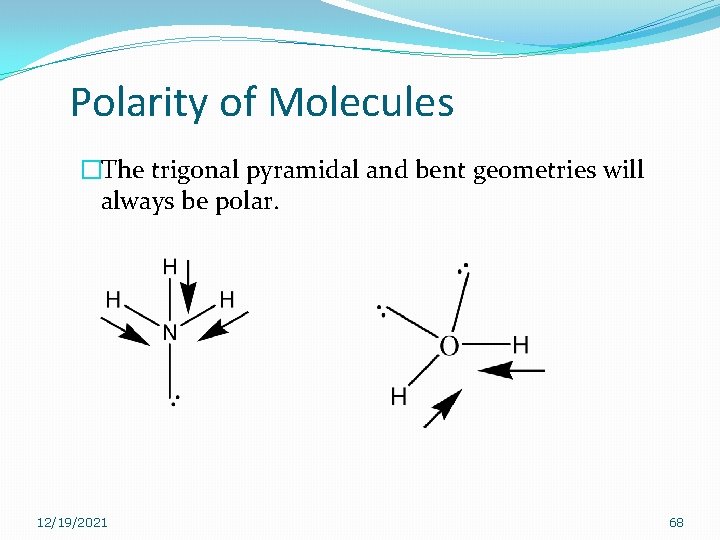
Polarity of Molecules �The trigonal pyramidal and bent geometries will always be polar. 12/19/2021 68
Summary �Molecules are non-polar when they have geometries of linear, trigonal planar, or tetrahedral AND all of the external atoms are the same. �Molecules are polar when they have different external atoms or have geometries of trigonal pyramidal and bent. 12/19/2021 69
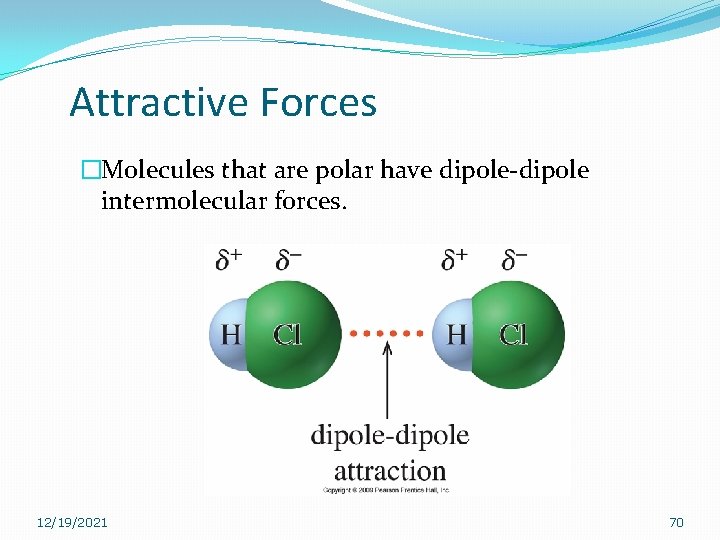
Attractive Forces �Molecules that are polar have dipole-dipole intermolecular forces. 12/19/2021 70

Attractive Forces �In a few cases, the dipole-dipole force is exceptionally strong. �This occurs when Hydrogen is bonded to either N, O, or F and the N, O, or F atom has one or more lone pairs. �This interaction is called Hydrogen Bonding. 12/19/2021 71
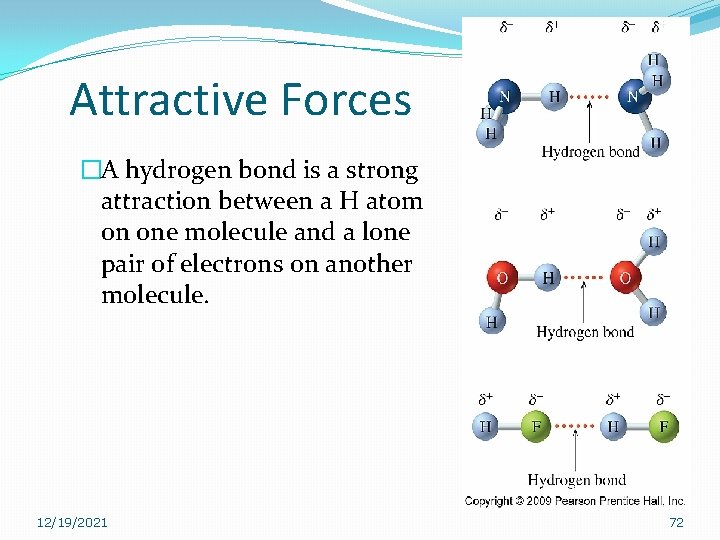
Attractive Forces �A hydrogen bond is a strong attraction between a H atom on one molecule and a lone pair of electrons on another molecule. 12/19/2021 72

Attractive Forces �Non-polar molecules have very weak London Dispersion forces. �These forces are due to the fact that electrons in our atoms are constantly moving and occasionally produce a brief dipole when they are not evenly distributed. 12/19/2021 73
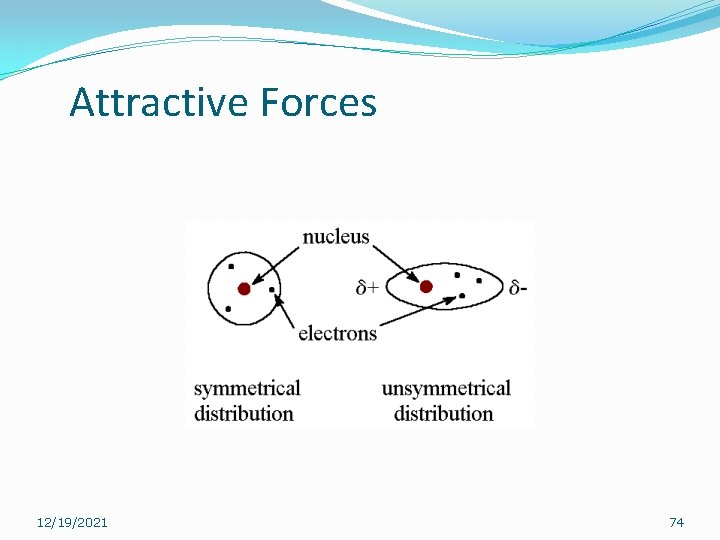
Attractive Forces 12/19/2021 74
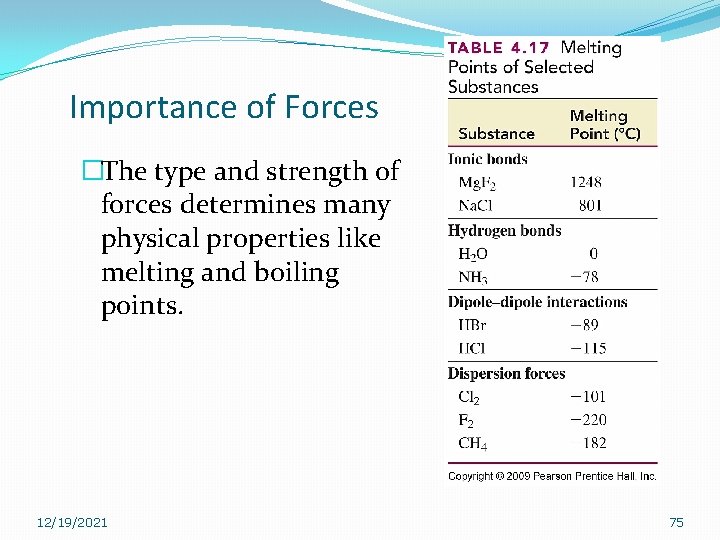
Importance of Forces �The type and strength of forces determines many physical properties like melting and boiling points. 12/19/2021 75
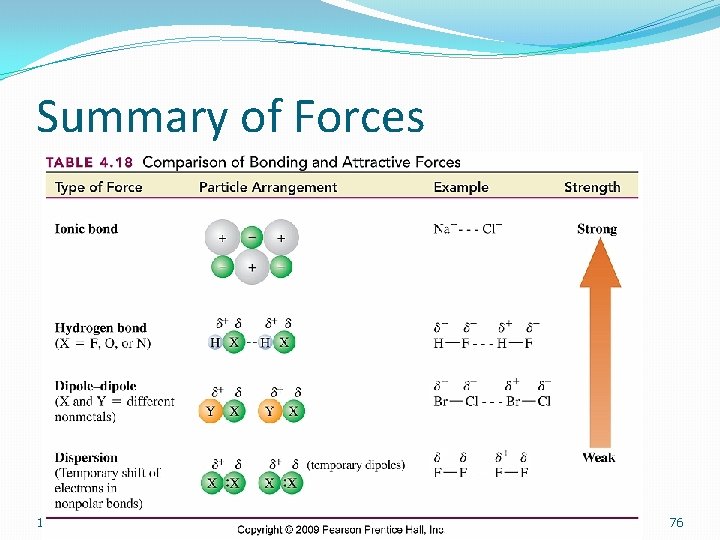
Summary of Forces 12/19/2021 76

Learning Check �What types of forces would each of the following substances have? �Cl 2 �HF �KF �OF 2 12/19/2021 77
- Slides: 77