Chapter 4 Compounds and Their Bonds 4 5
Chapter 4 Compounds and Their Bonds 4. 5 Covalent Compounds Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 1
Covalent Bonds Covalent bonds form • when atoms share electrons to complete octets. • between two nonmetal atoms. • between nonmetal atoms from Groups 4 A(14), 5 A(15), 6 A(16), and 7 A(17). 2

Hydrogen Molecule A hydrogen molecule • is stable with two electrons (helium). • has a shared pair of electrons. 3
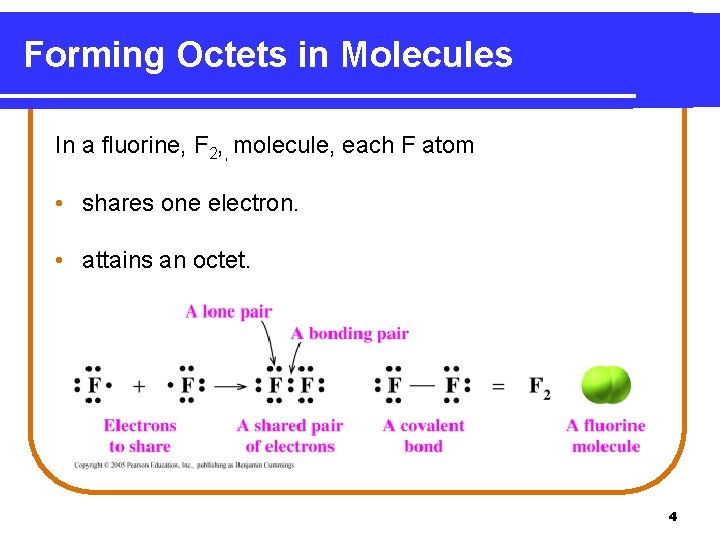
Forming Octets in Molecules In a fluorine, F 2, , molecule, each F atom • shares one electron. • attains an octet. 4
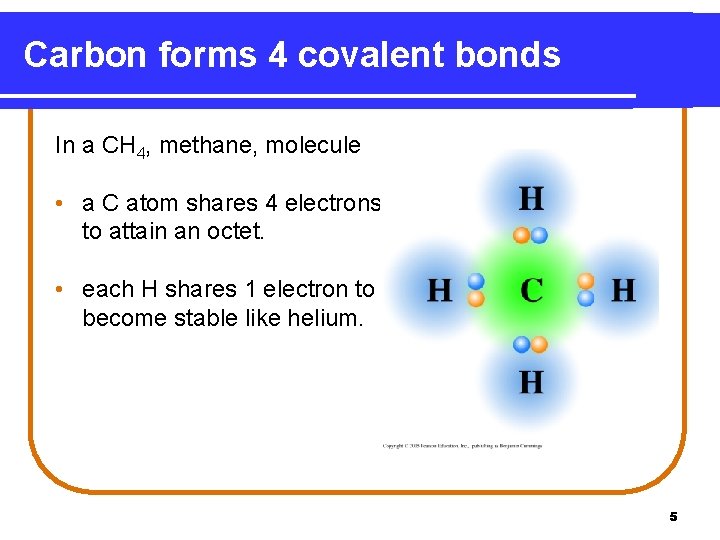
Carbon forms 4 covalent bonds In a CH 4, methane, molecule • a C atom shares 4 electrons to attain an octet. • each H shares 1 electron to become stable like helium. 5
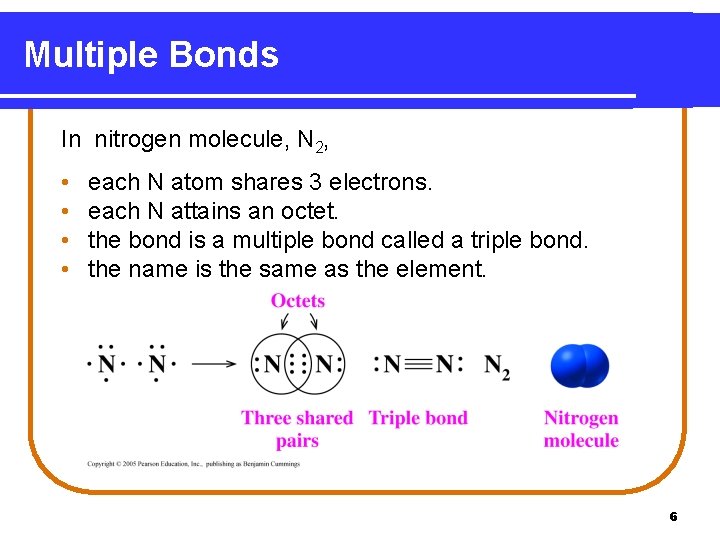
Multiple Bonds In nitrogen molecule, N 2, • • each N atom shares 3 electrons. each N attains an octet. the bond is a multiple bond called a triple bond. the name is the same as the element. 6
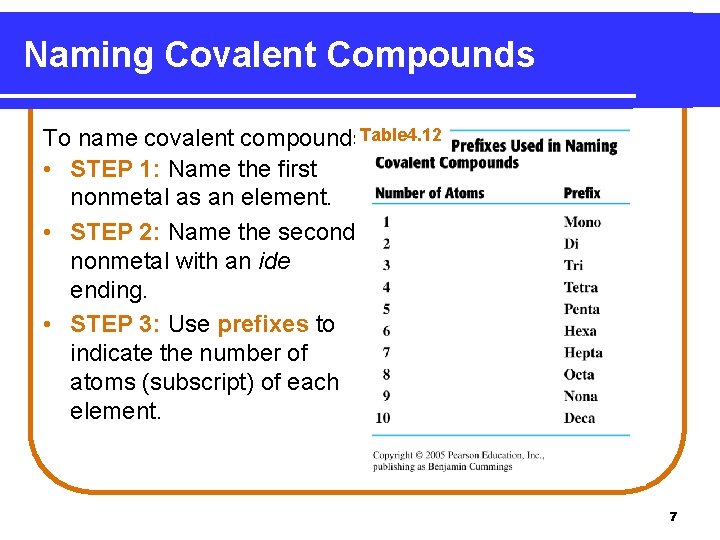
Naming Covalent Compounds To name covalent compounds. Table 4. 12 • STEP 1: Name the first nonmetal as an element. • STEP 2: Name the second nonmetal with an ide ending. • STEP 3: Use prefixes to indicate the number of atoms (subscript) of each element. 7
Naming Covalent Compounds What is the name of SO 3? 1. The first nonmetal is S sulfur. 2. The second nonmetal is O named oxide. 3. The subscript 3 of O is shown as the prefix tri. SO 3 sulfur trioxide The subscript 1 (for S) or mono is understood. 8
Naming Covalent Compounds Name P 4 S 3. 1. The first nonmetal P is phosphorus. 2. The second nonmetal S is sulfide. 3. The subscript 4 of P is shown as tetra. The subscript 3 of O is shown as tri. P 4 S 3 tetraphosphorus trisulfide 9
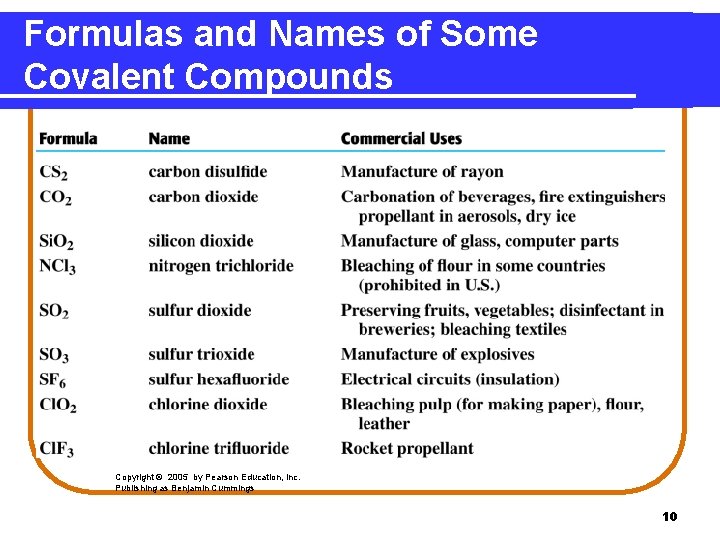
Formulas and Names of Some Covalent Compounds Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 10
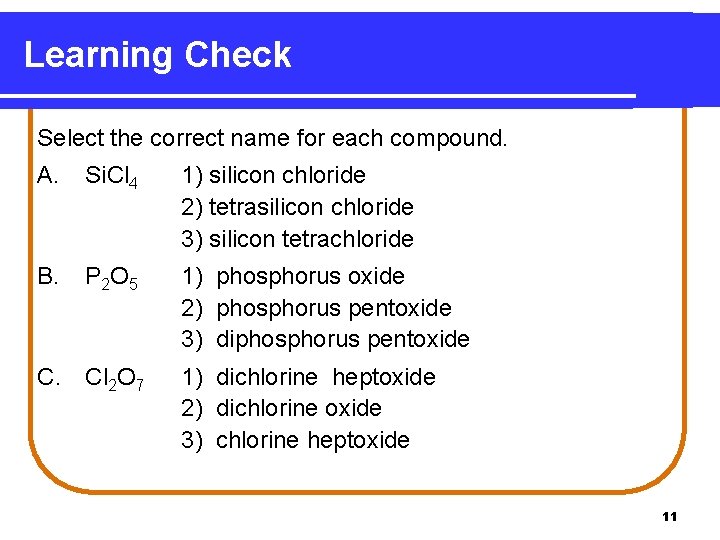
Learning Check Select the correct name for each compound. A. Si. Cl 4 1) silicon chloride 2) tetrasilicon chloride 3) silicon tetrachloride B. P 2 O 5 1) phosphorus oxide 2) phosphorus pentoxide 3) diphosphorus pentoxide C. Cl 2 O 7 1) dichlorine heptoxide 2) dichlorine oxide 3) chlorine heptoxide 11
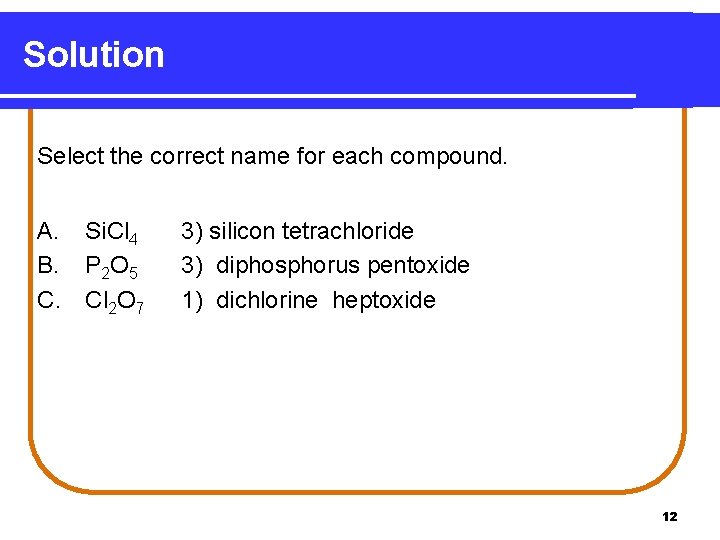
Solution Select the correct name for each compound. A. B. C. Si. Cl 4 P 2 O 5 Cl 2 O 7 3) silicon tetrachloride 3) diphosphorus pentoxide 1) dichlorine heptoxide 12
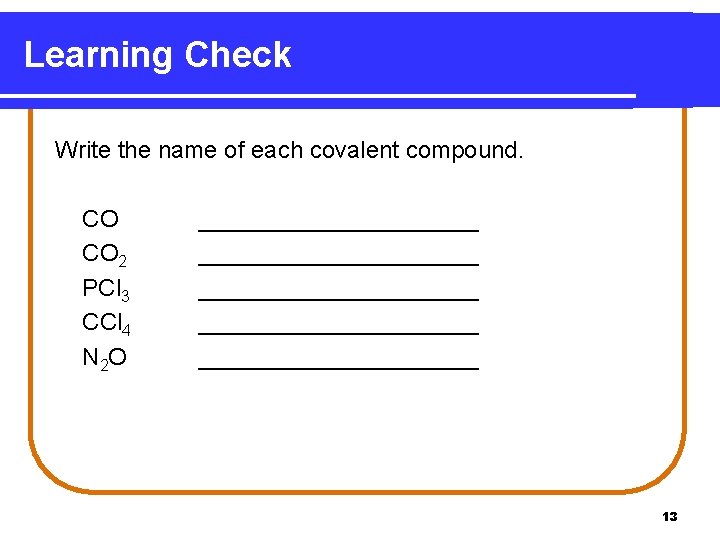
Learning Check Write the name of each covalent compound. CO CO 2 PCl 3 CCl 4 N 2 O _____________________ ___________ 13
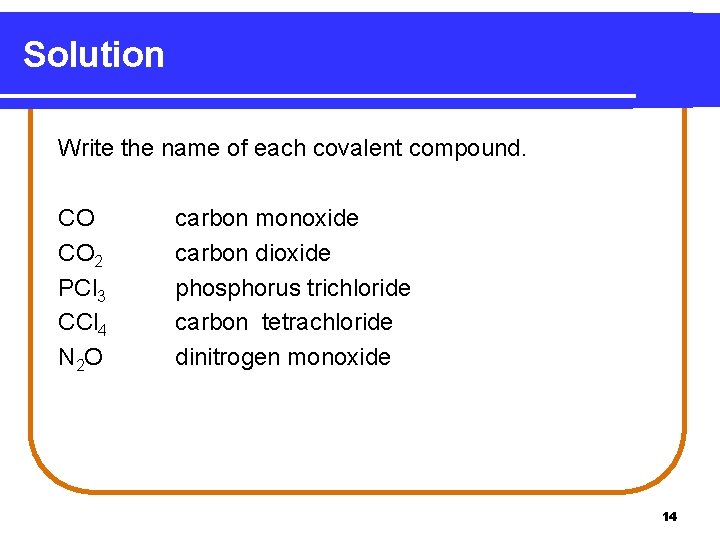
Solution Write the name of each covalent compound. CO CO 2 PCl 3 CCl 4 N 2 O carbon monoxide carbon dioxide phosphorus trichloride carbon tetrachloride dinitrogen monoxide 14
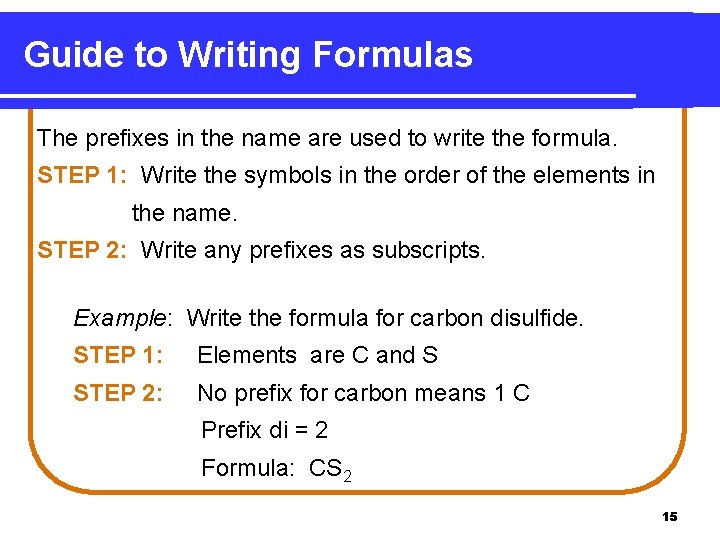
Guide to Writing Formulas The prefixes in the name are used to write the formula. STEP 1: Write the symbols in the order of the elements in the name. STEP 2: Write any prefixes as subscripts. Example: Write the formula for carbon disulfide. STEP 1: Elements are C and S STEP 2: No prefix for carbon means 1 C Prefix di = 2 Formula: CS 2 15
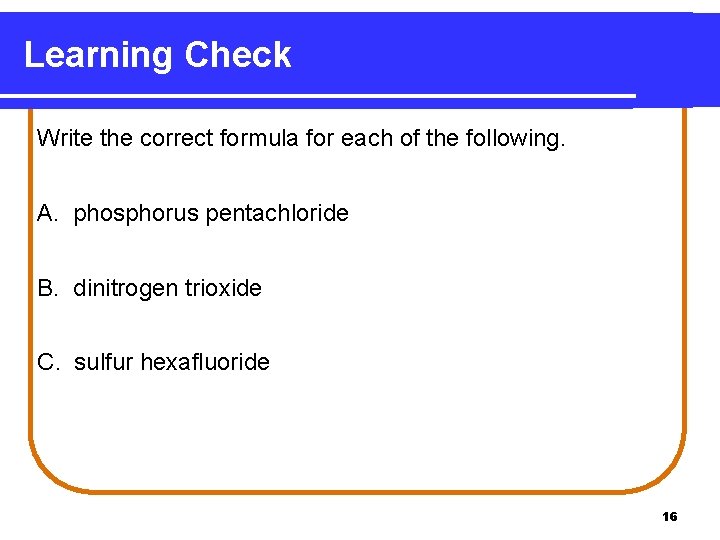
Learning Check Write the correct formula for each of the following. A. phosphorus pentachloride B. dinitrogen trioxide C. sulfur hexafluoride 16
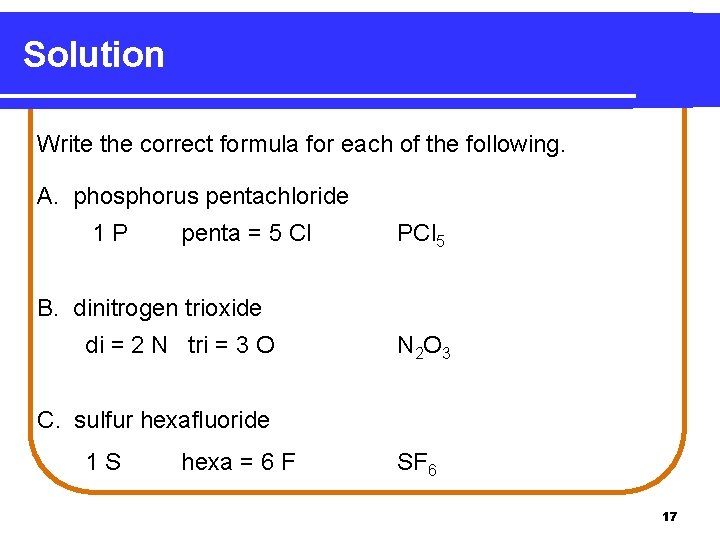
Solution Write the correct formula for each of the following. A. phosphorus pentachloride 1 P penta = 5 Cl PCl 5 B. dinitrogen trioxide di = 2 N tri = 3 O N 2 O 3 C. sulfur hexafluoride 1 S hexa = 6 F SF 6 17
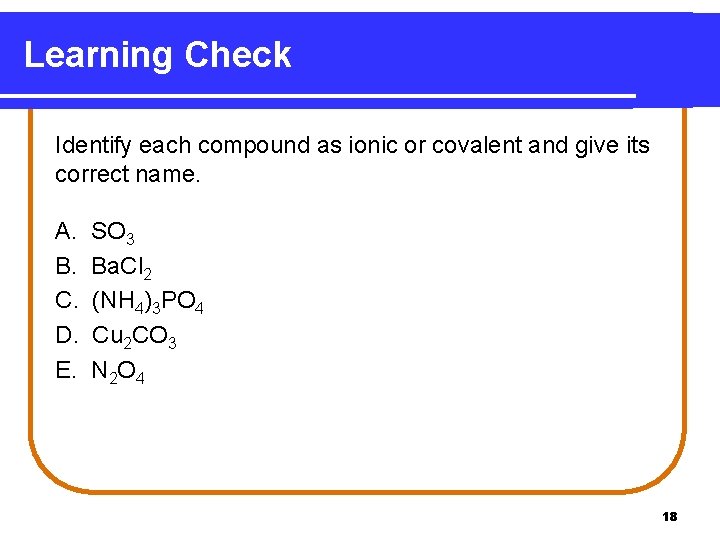
Learning Check Identify each compound as ionic or covalent and give its correct name. A. B. C. D. E. SO 3 Ba. Cl 2 (NH 4)3 PO 4 Cu 2 CO 3 N 2 O 4 18
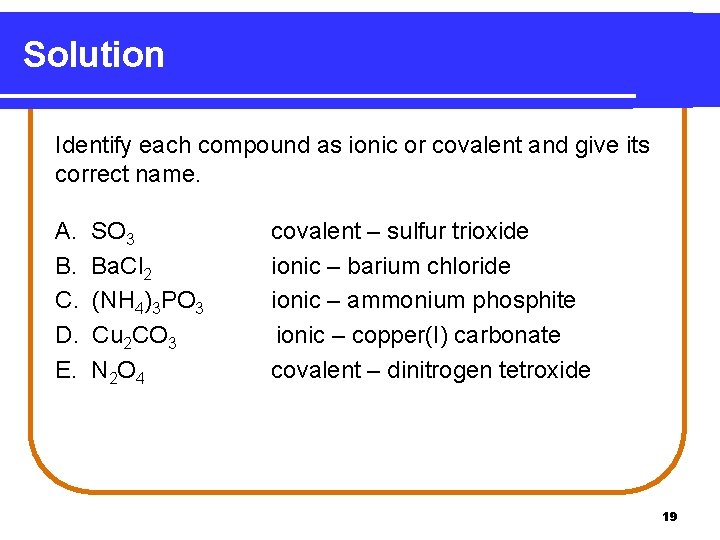
Solution Identify each compound as ionic or covalent and give its correct name. A. B. C. D. E. SO 3 Ba. Cl 2 (NH 4)3 PO 3 Cu 2 CO 3 N 2 O 4 covalent – sulfur trioxide ionic – barium chloride ionic – ammonium phosphite ionic – copper(I) carbonate covalent – dinitrogen tetroxide 19
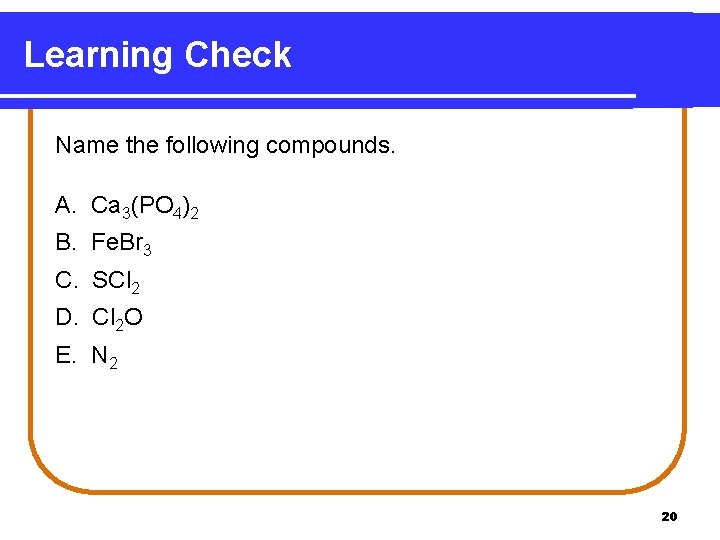
Learning Check Name the following compounds. A. Ca 3(PO 4)2 B. Fe. Br 3 C. SCl 2 D. Cl 2 O E. N 2 20
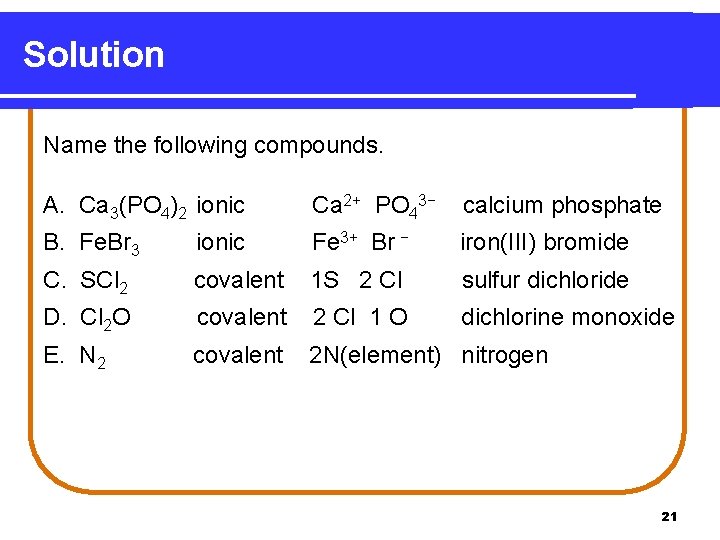
Solution Name the following compounds. A. Ca 3(PO 4)2 ionic Ca 2+ PO 43− calcium phosphate B. Fe. Br 3 ionic Fe 3+ Br − iron(III) bromide C. SCl 2 covalent 1 S 2 Cl sulfur dichloride D. Cl 2 O covalent 2 Cl 1 O dichlorine monoxide E. N 2 covalent 2 N(element) nitrogen 21
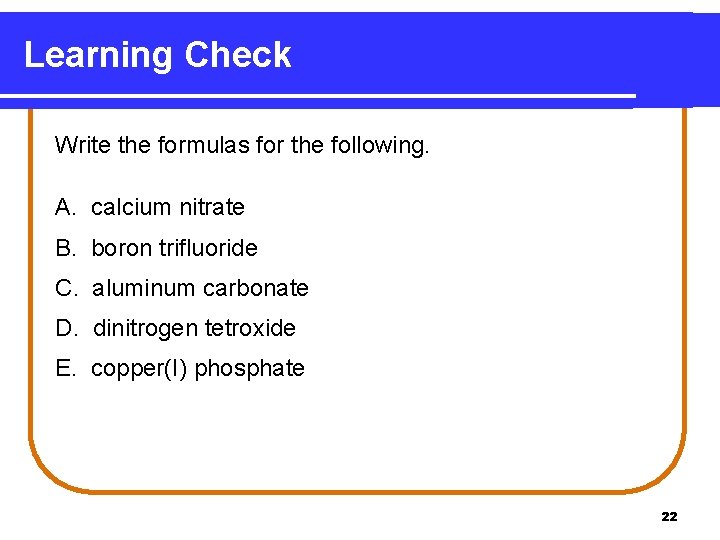
Learning Check Write the formulas for the following. A. calcium nitrate B. boron trifluoride C. aluminum carbonate D. dinitrogen tetroxide E. copper(I) phosphate 22
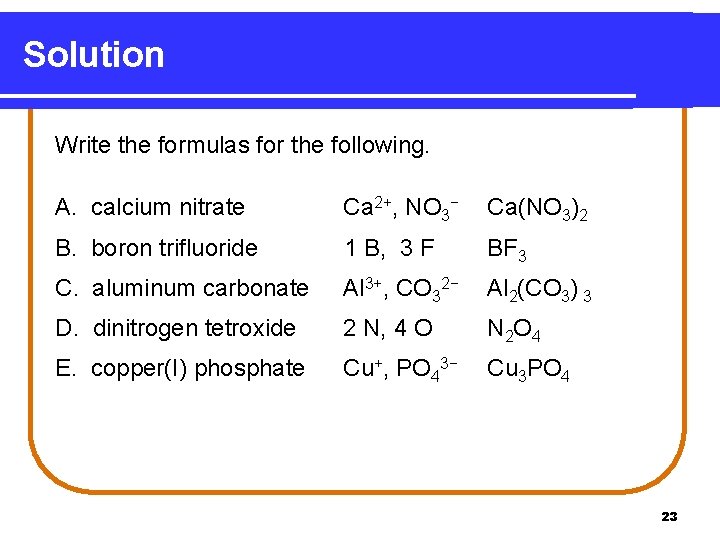
Solution Write the formulas for the following. A. calcium nitrate Ca 2+, NO 3− Ca(NO 3)2 B. boron trifluoride 1 B, 3 F BF 3 C. aluminum carbonate Al 3+, CO 32− Al 2(CO 3) 3 D. dinitrogen tetroxide 2 N, 4 O N 2 O 4 E. copper(I) phosphate Cu+, PO 43− Cu 3 PO 4 23

Predicting Compounds using Lewis Dot Structures l Going back to the idea of Lewis dot configuration as a good way to keep track of valence electrons for predicting structure of ionic/covalent compounds. 24
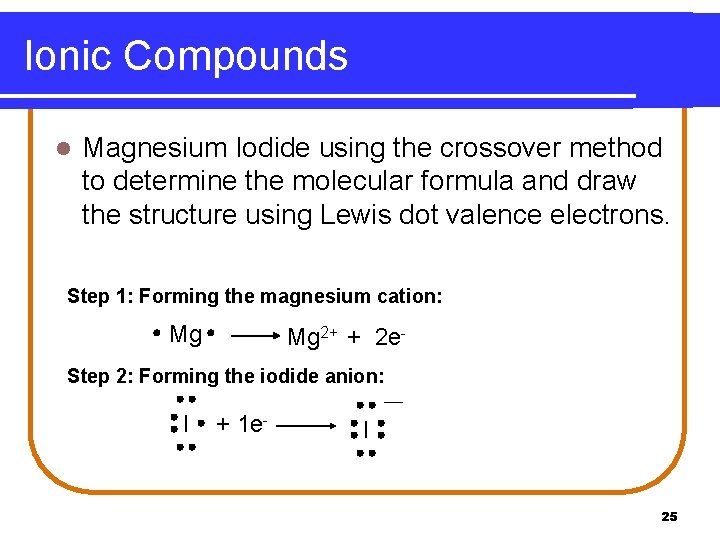
Ionic Compounds l Magnesium Iodide using the crossover method to determine the molecular formula and draw the structure using Lewis dot valence electrons. Step 1: Forming the magnesium cation: Mg Mg 2+ + 2 e- Step 2: Forming the iodide anion: I + 1 e- I 25

Step 3: Putting the ions together We need 2 iodide anions to balance the +2 charge on the magnesium, as indicated by the formula Mg. I 2 I Mg 2+ I 26
Covalent Compounds l Covalent compounds between oxygen and hydrogen Step 1: Determine how many bonds are formed by oxygen Step 2: Determine how many hydrogen atoms are in the chemical formula (hydrogen forms a single bond) Step 3: Draw the structure 27
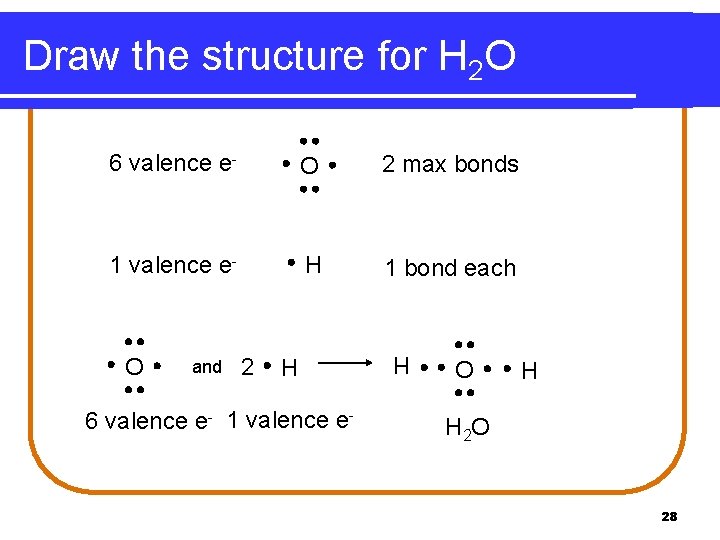
Draw the structure for H 2 O 6 valence e- O 2 max bonds 1 valence e- H 1 bond each and 2 H 6 valence e- 1 valence e- H O O H H 2 O 28
Covalent Compounds l Covalent compounds between carbon and hydrogen Step 1: Determine how many bonds are formed by carbon Step 2: Determine how many hydrogen atoms are in the chemical formula (hydrogen forms a single bond) Step 3: Draw the structure 29
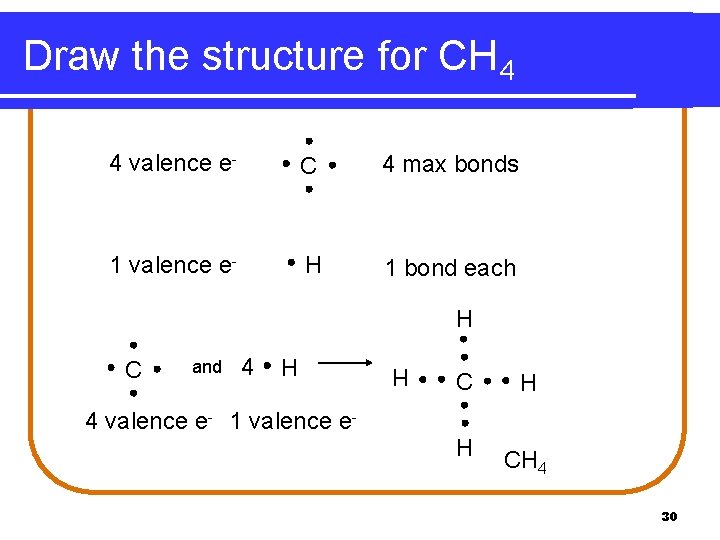
Draw the structure for CH 4 4 valence e- C 4 max bonds 1 valence e- H 1 bond each H and 4 H H C C H 4 valence e- 1 valence e. H CH 4 30
- Slides: 30