Chapter 4 Compounds and Their Bonds 4 4

Chapter 4 Compounds and Their Bonds 4. 4 Polyatomic Ions Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 1
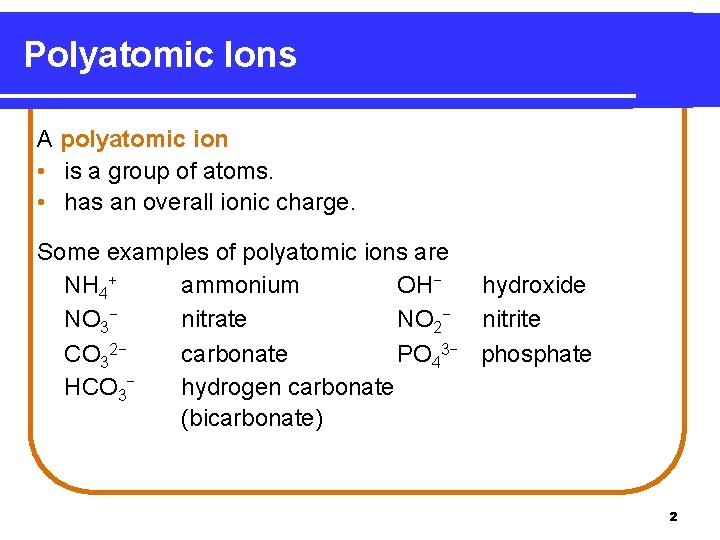
Polyatomic Ions A polyatomic ion • is a group of atoms. • has an overall ionic charge. Some examples of polyatomic ions are NH 4+ ammonium OH− hydroxide NO 3− nitrate NO 2− nitrite CO 32− carbonate PO 43− phosphate HCO 3− hydrogen carbonate (bicarbonate) 2
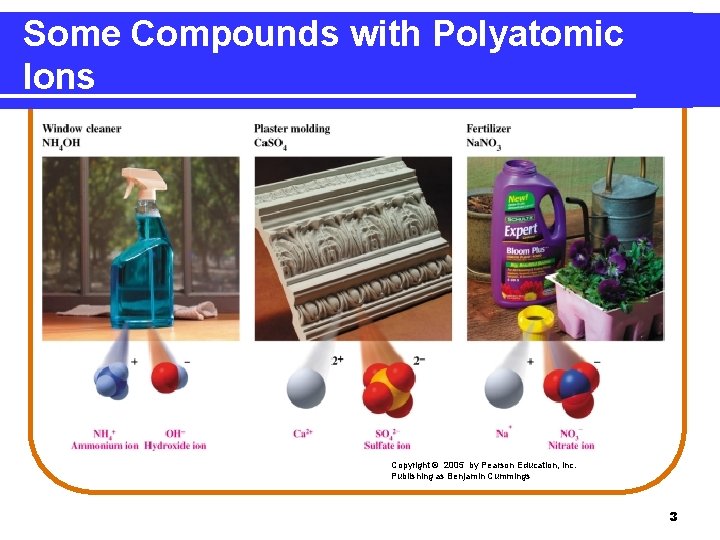
Some Compounds with Polyatomic Ions Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 3
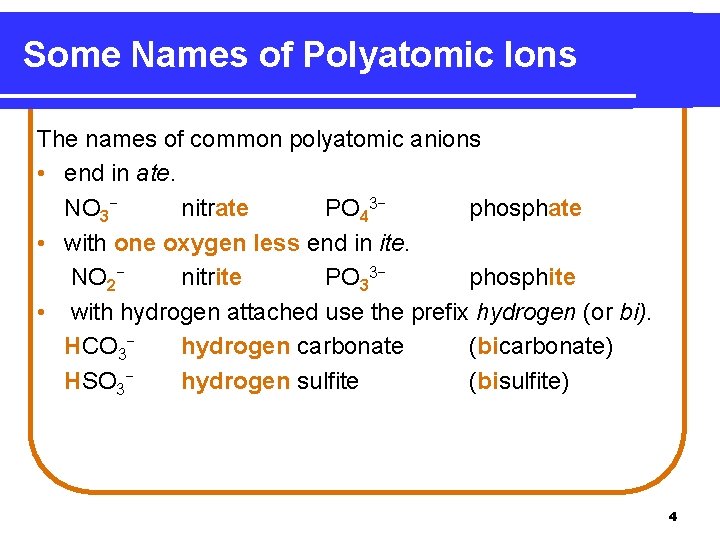
Some Names of Polyatomic Ions The names of common polyatomic anions • end in ate. NO 3− nitrate PO 43− phosphate • with one oxygen less end in ite. NO 2− nitrite PO 33− phosphite • with hydrogen attached use the prefix hydrogen (or bi). HCO 3− hydrogen carbonate (bicarbonate) HSO 3− hydrogen sulfite (bisulfite) 4
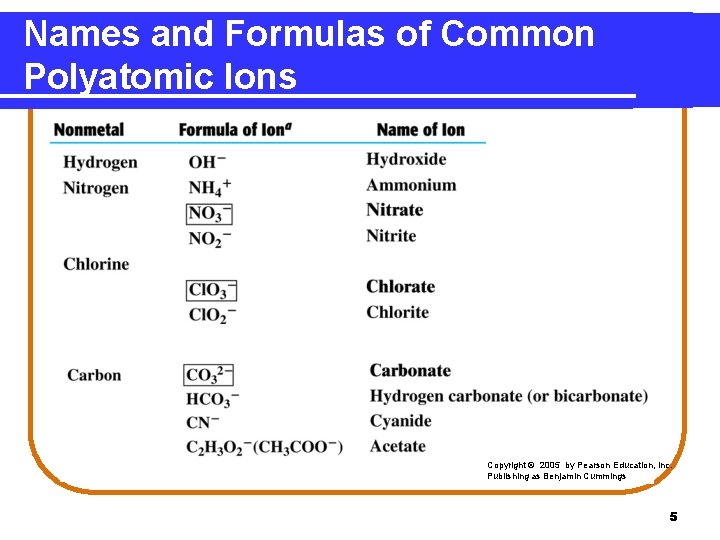
Names and Formulas of Common Polyatomic Ions Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 5
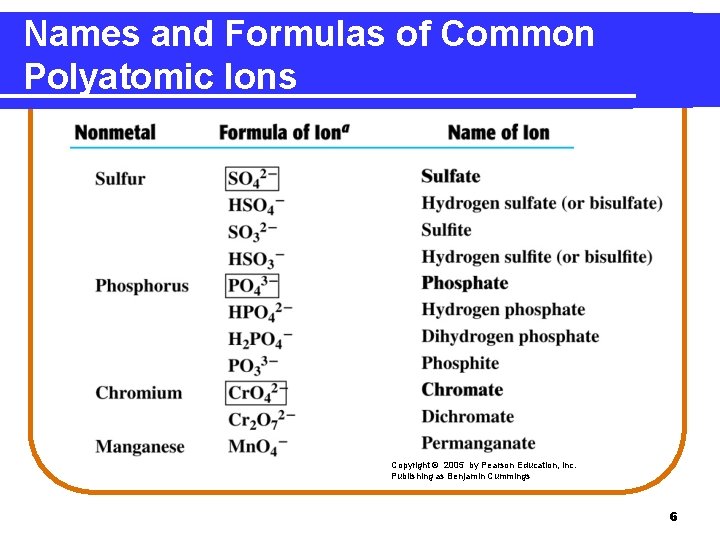
Names and Formulas of Common Polyatomic Ions Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 6
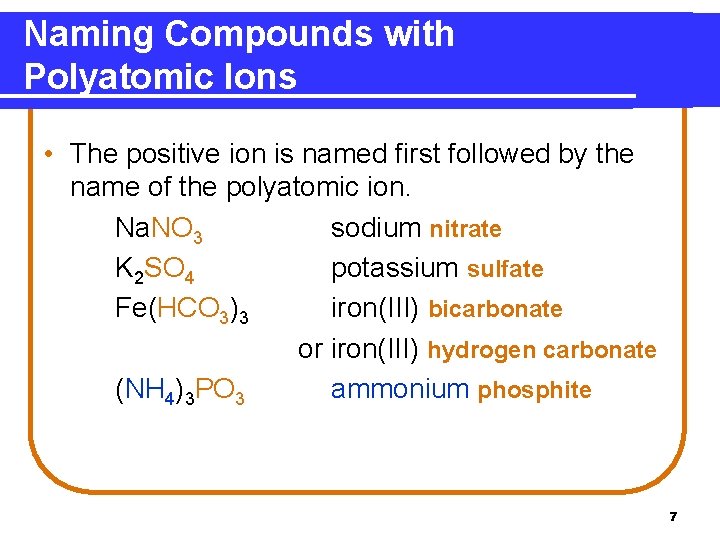
Naming Compounds with Polyatomic Ions • The positive ion is named first followed by the name of the polyatomic ion. Na. NO 3 sodium nitrate K 2 SO 4 potassium sulfate Fe(HCO 3)3 iron(III) bicarbonate or iron(III) hydrogen carbonate (NH 4)3 PO 3 ammonium phosphite 7
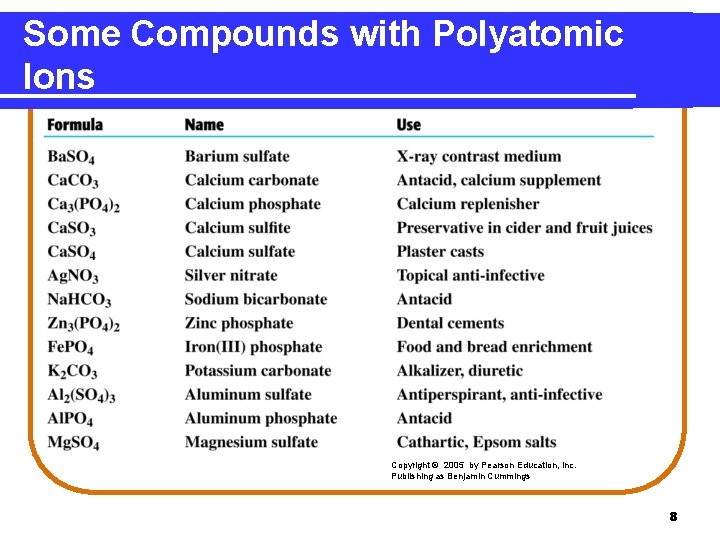
Some Compounds with Polyatomic Ions Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 8
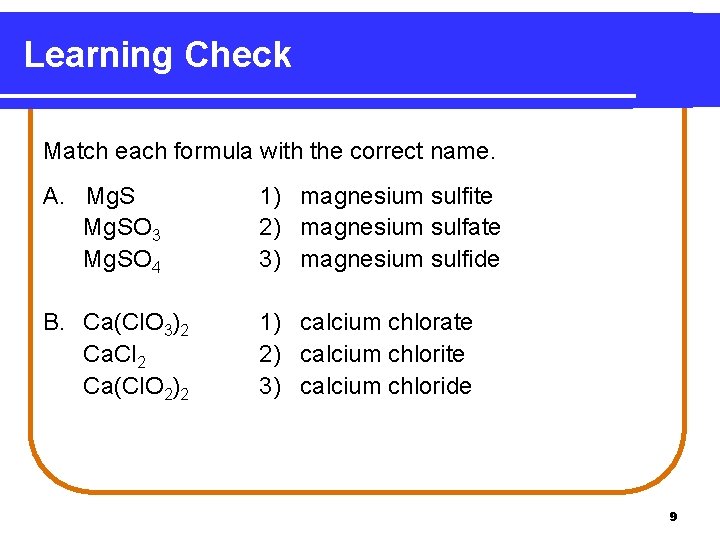
Learning Check Match each formula with the correct name. A. Mg. SO 3 Mg. SO 4 1) magnesium sulfite 2) magnesium sulfate 3) magnesium sulfide B. Ca(Cl. O 3)2 Ca. Cl 2 Ca(Cl. O 2)2 1) calcium chlorate 2) calcium chlorite 3) calcium chloride 9
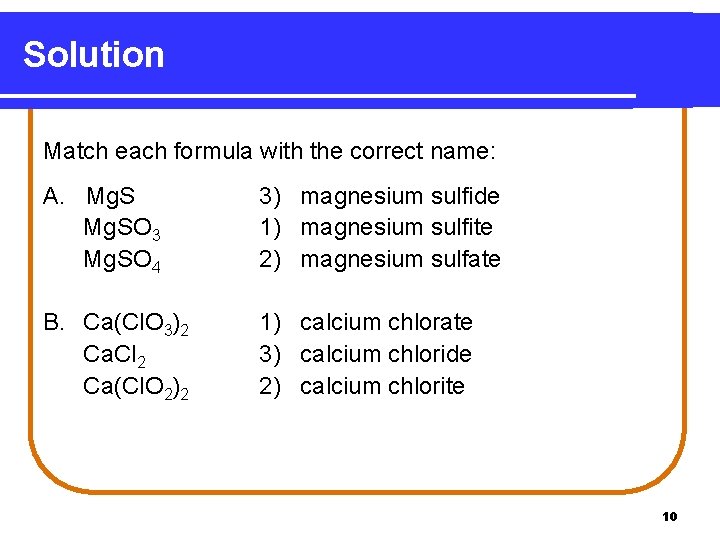
Solution Match each formula with the correct name: A. Mg. SO 3 Mg. SO 4 3) magnesium sulfide 1) magnesium sulfite 2) magnesium sulfate B. Ca(Cl. O 3)2 Ca. Cl 2 Ca(Cl. O 2)2 1) calcium chlorate 3) calcium chloride 2) calcium chlorite 10
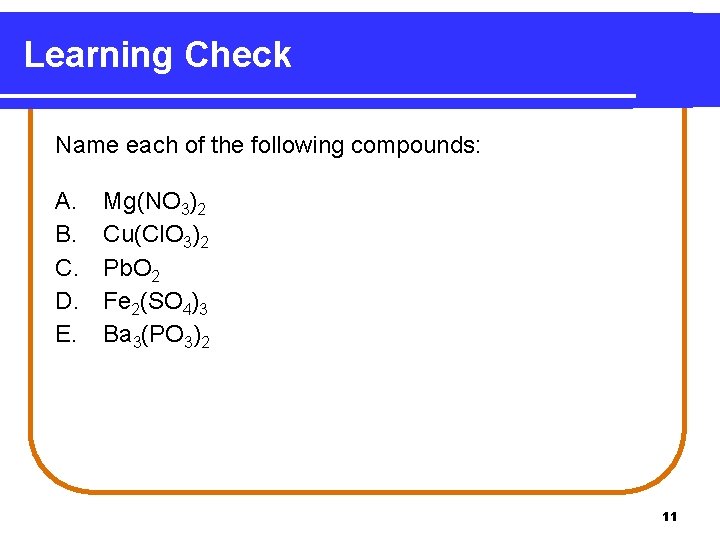
Learning Check Name each of the following compounds: A. B. C. D. E. Mg(NO 3)2 Cu(Cl. O 3)2 Pb. O 2 Fe 2(SO 4)3 Ba 3(PO 3)2 11
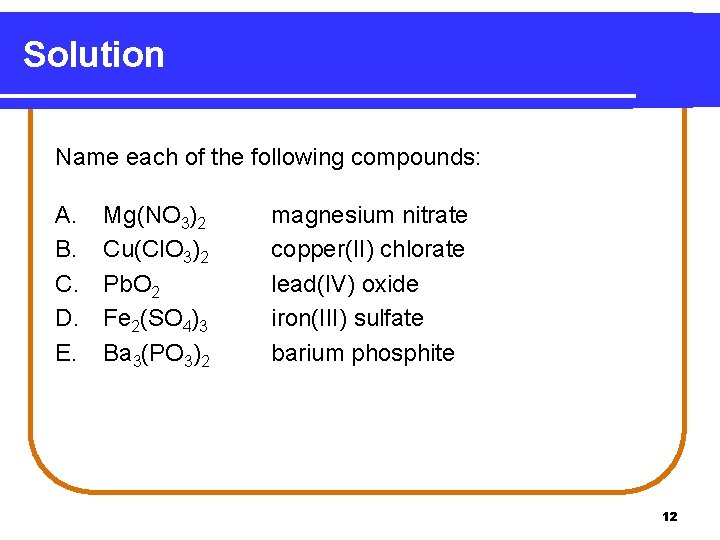
Solution Name each of the following compounds: A. B. C. D. E. Mg(NO 3)2 Cu(Cl. O 3)2 Pb. O 2 Fe 2(SO 4)3 Ba 3(PO 3)2 magnesium nitrate copper(II) chlorate lead(IV) oxide iron(III) sulfate barium phosphite 12
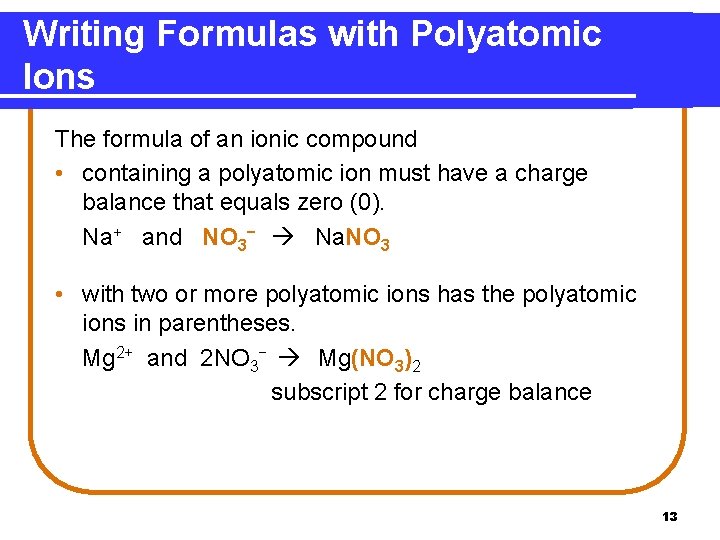
Writing Formulas with Polyatomic Ions The formula of an ionic compound • containing a polyatomic ion must have a charge balance that equals zero (0). Na+ and NO 3− Na. NO 3 • with two or more polyatomic ions has the polyatomic ions in parentheses. Mg 2+ and 2 NO 3− Mg(NO 3)2 subscript 2 for charge balance 13
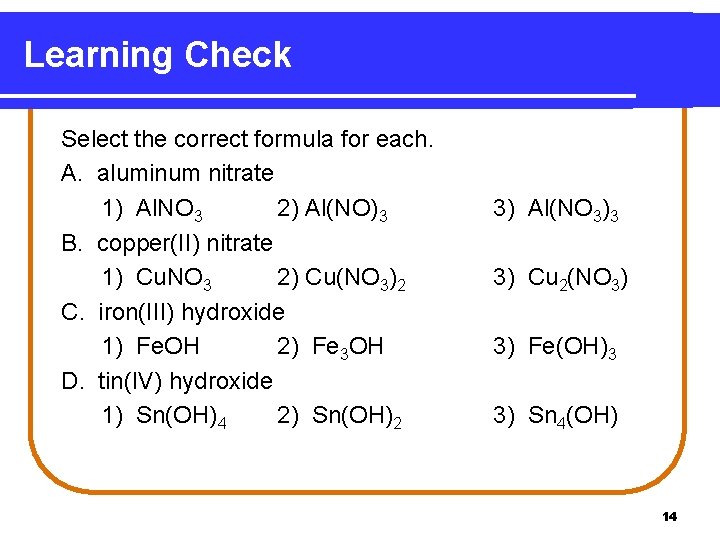
Learning Check Select the correct formula for each. A. aluminum nitrate 1) Al. NO 3 2) Al(NO)3 B. copper(II) nitrate 1) Cu. NO 3 2) Cu(NO 3)2 C. iron(III) hydroxide 1) Fe. OH 2) Fe 3 OH D. tin(IV) hydroxide 1) Sn(OH)4 2) Sn(OH)2 3) Al(NO 3)3 3) Cu 2(NO 3) 3) Fe(OH)3 3) Sn 4(OH) 14
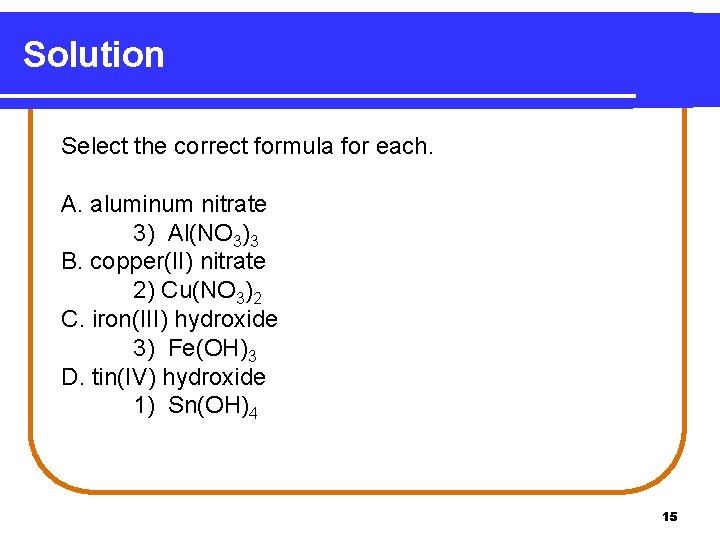
Solution Select the correct formula for each. A. aluminum nitrate 3) Al(NO 3)3 B. copper(II) nitrate 2) Cu(NO 3)2 C. iron(III) hydroxide 3) Fe(OH)3 D. tin(IV) hydroxide 1) Sn(OH)4 15
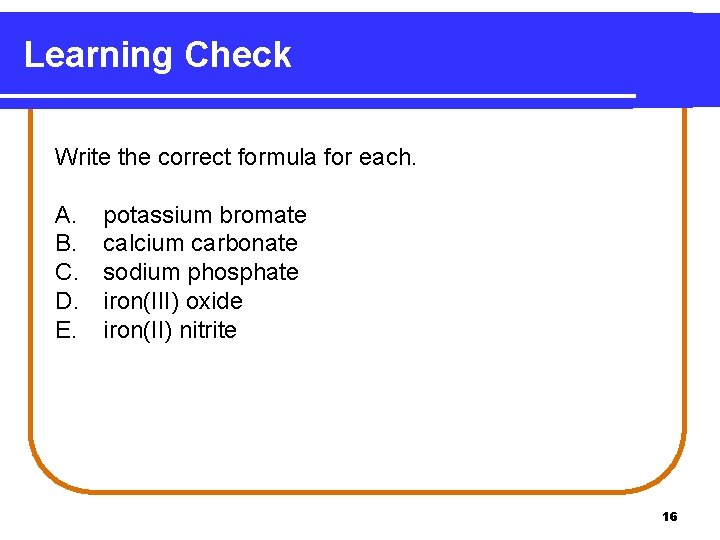
Learning Check Write the correct formula for each. A. B. C. D. E. potassium bromate calcium carbonate sodium phosphate iron(III) oxide iron(II) nitrite 16
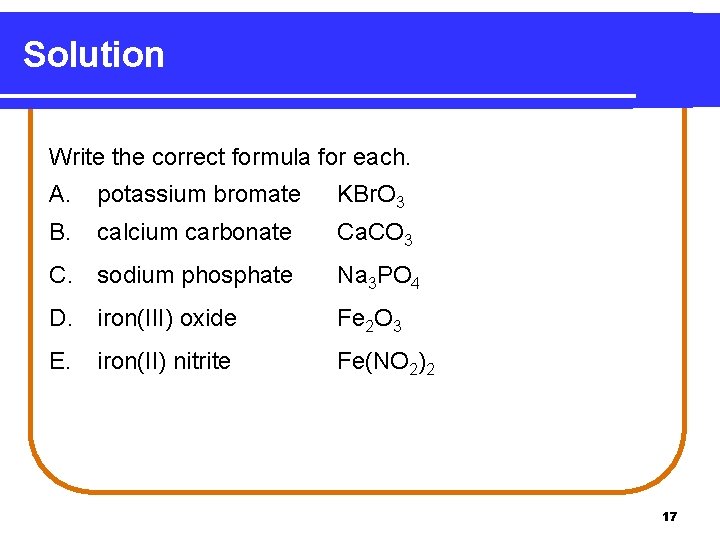
Solution Write the correct formula for each. A. potassium bromate KBr. O 3 B. calcium carbonate Ca. CO 3 C. sodium phosphate Na 3 PO 4 D. iron(III) oxide Fe 2 O 3 E. iron(II) nitrite Fe(NO 2)2 17
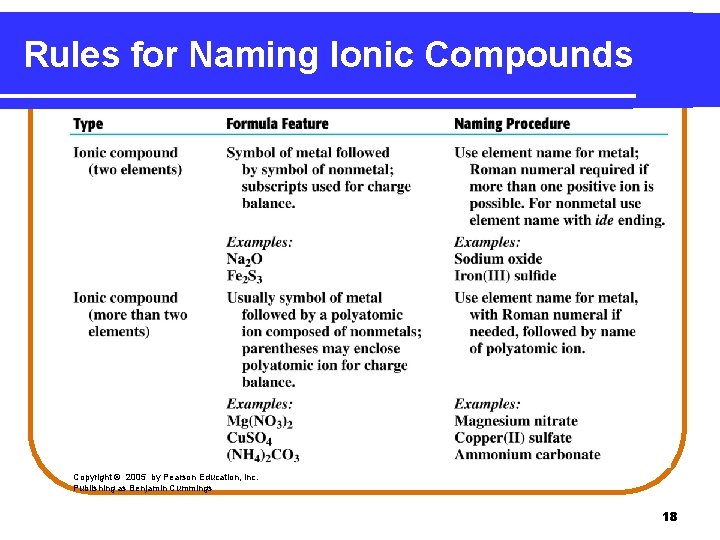
Rules for Naming Ionic Compounds Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 18
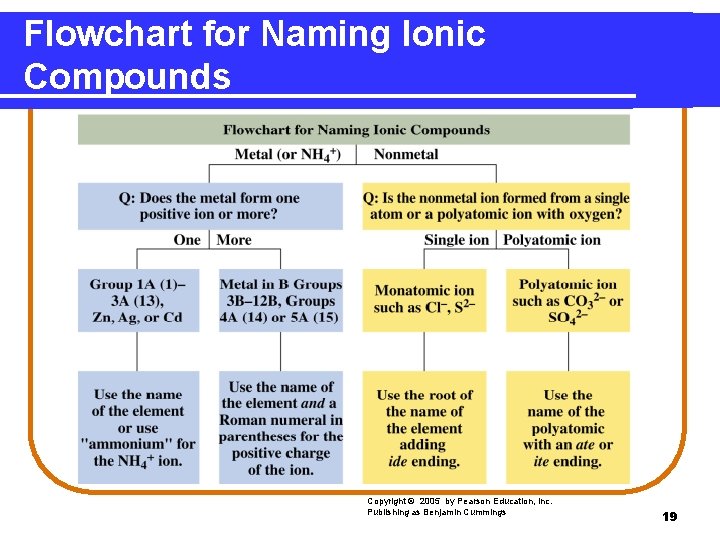
Flowchart for Naming Ionic Compounds Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 19
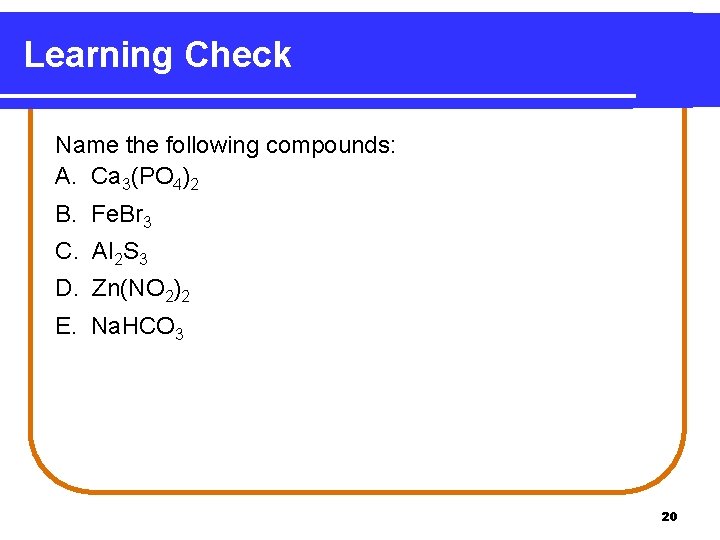
Learning Check Name the following compounds: A. Ca 3(PO 4)2 B. Fe. Br 3 C. Al 2 S 3 D. Zn(NO 2)2 E. Na. HCO 3 20
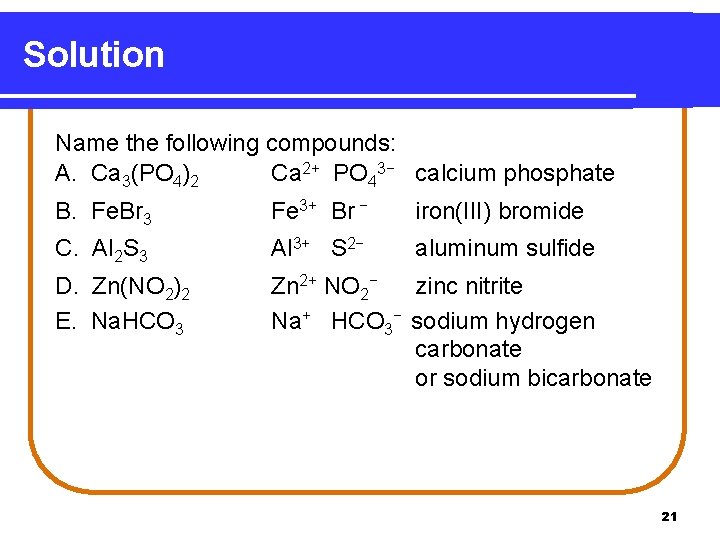
Solution Name the following compounds: A. Ca 3(PO 4)2 Ca 2+ PO 43− calcium phosphate B. Fe. Br 3 Fe 3+ Br − iron(III) bromide C. Al 2 S 3 Al 3+ S 2− aluminum sulfide D. Zn(NO 2)2 E. Na. HCO 3 Zn 2+ NO 2− zinc nitrite Na+ HCO 3− sodium hydrogen carbonate or sodium bicarbonate 21
Learning Check Write the formulas for the following: A. calcium nitrate B. iron(II) hydroxide C. aluminum carbonate D. copper(II) bromide E. lithium phosphate 22
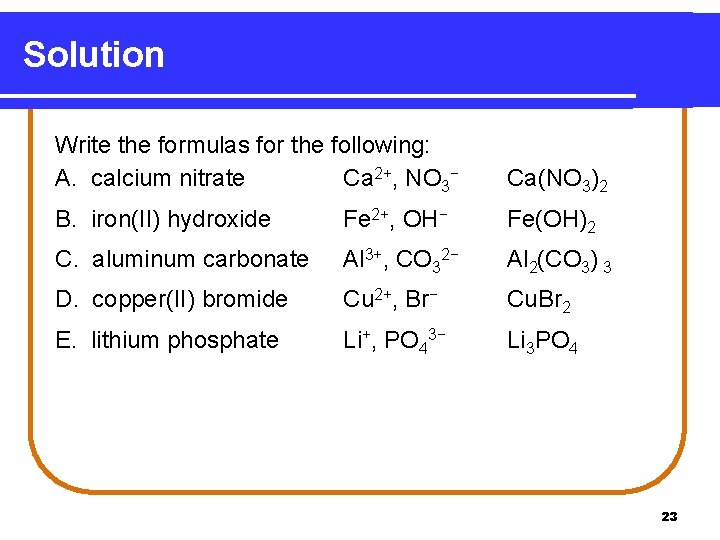
Solution Write the formulas for the following: A. calcium nitrate Ca 2+, NO 3− Ca(NO 3)2 B. iron(II) hydroxide Fe 2+, OH− Fe(OH)2 C. aluminum carbonate Al 3+, CO 32− Al 2(CO 3) 3 D. copper(II) bromide Cu 2+, Br− Cu. Br 2 E. lithium phosphate Li+, PO 43− Li 3 PO 4 23
- Slides: 23