Chapter 4 Chemical Bonding the Ionic Bond Model
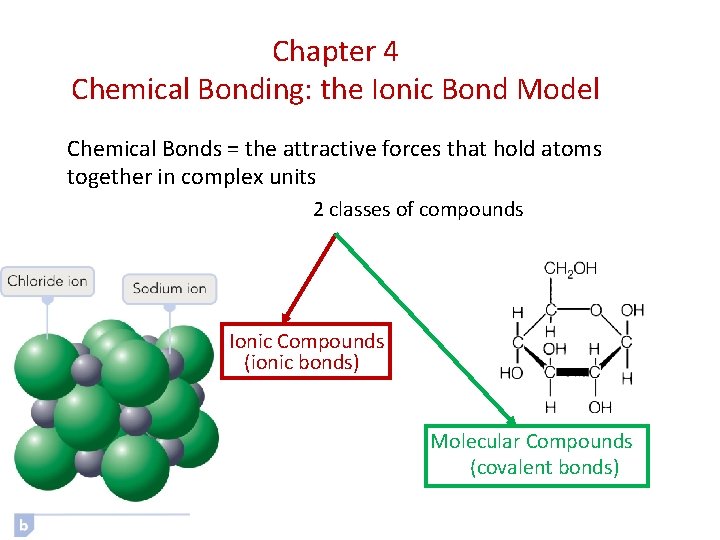
Chapter 4 Chemical Bonding: the Ionic Bond Model Chemical Bonds = the attractive forces that hold atoms together in complex units 2 classes of compounds Ionic Compounds (ionic bonds) Molecular Compounds (covalent bonds)
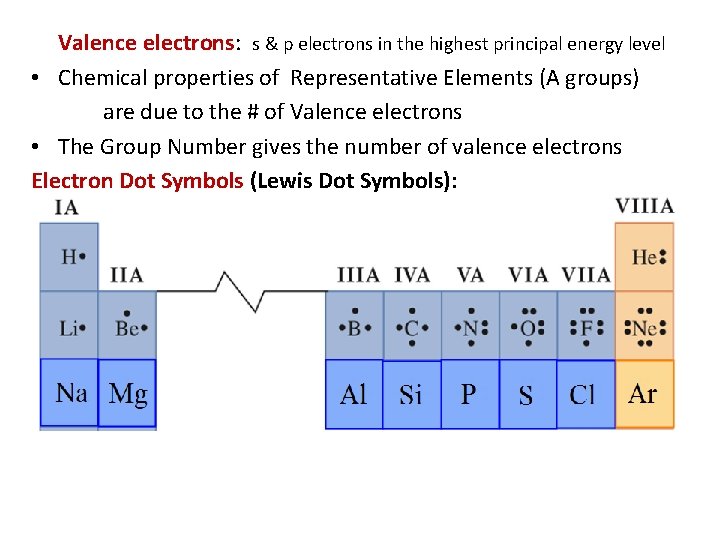
Valence electrons: s & p electrons in the highest principal energy level • Chemical properties of Representative Elements (A groups) are due to the # of Valence electrons • The Group Number gives the number of valence electrons Electron Dot Symbols (Lewis Dot Symbols):
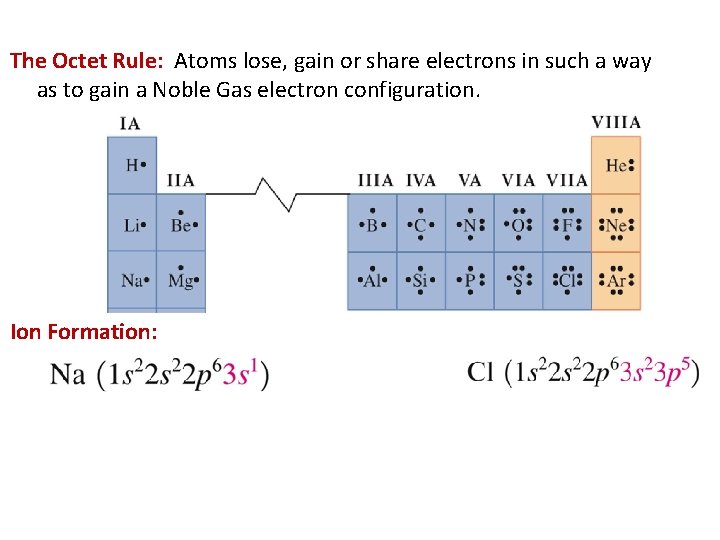
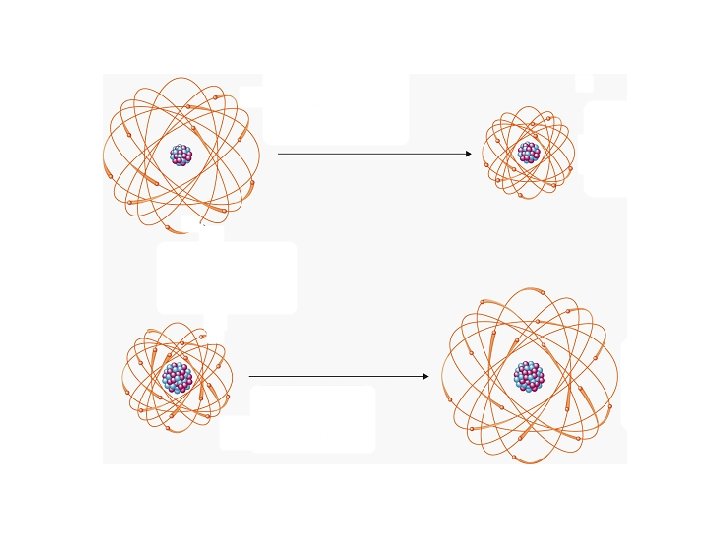
The Octet Rule: Atoms lose, gain or share electrons in such a way as to gain a Noble Gas electron configuration. Ion Formation:
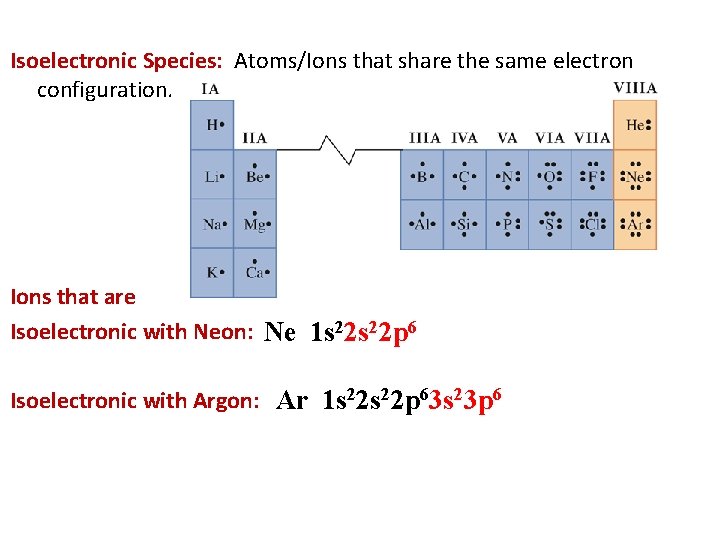
Isoelectronic Species: Atoms/Ions that share the same electron configuration. Ions that are Isoelectronic with Neon: Ne 1 s 22 p 6 Isoelectronic with Argon: Ar 1 s 22 p 63 s 23 p 6
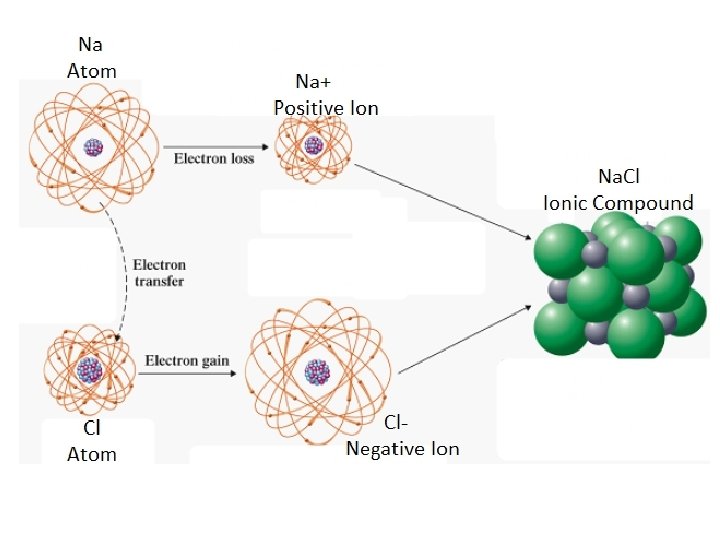
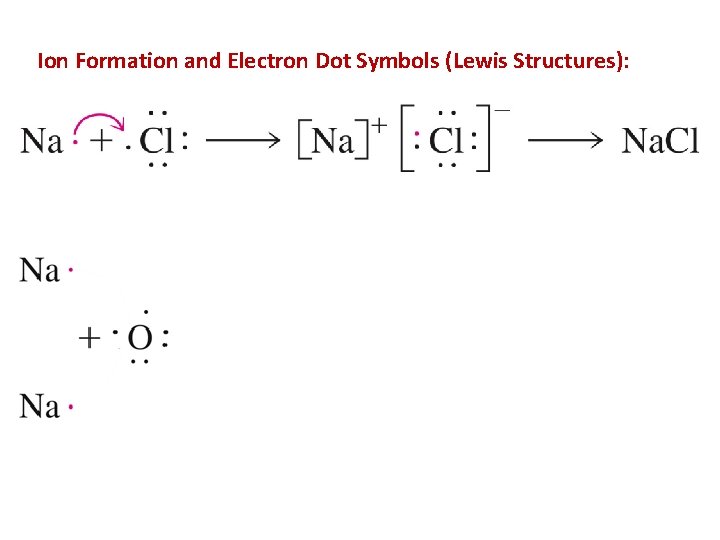
Ion Formation and Electron Dot Symbols (Lewis Structures):
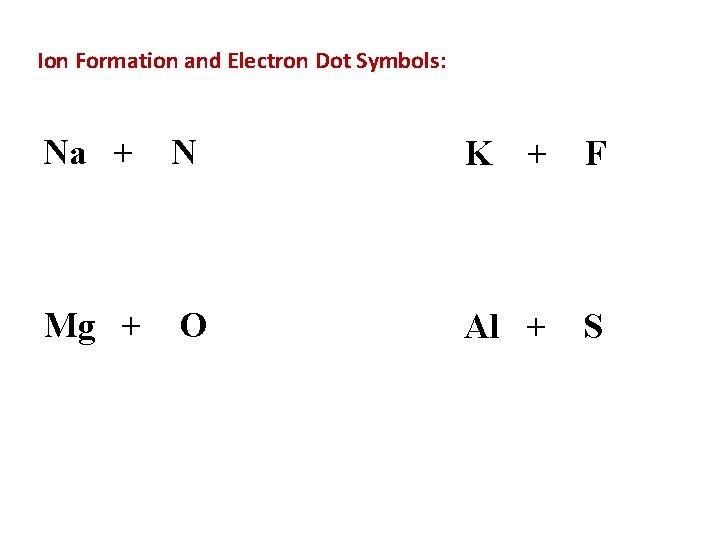
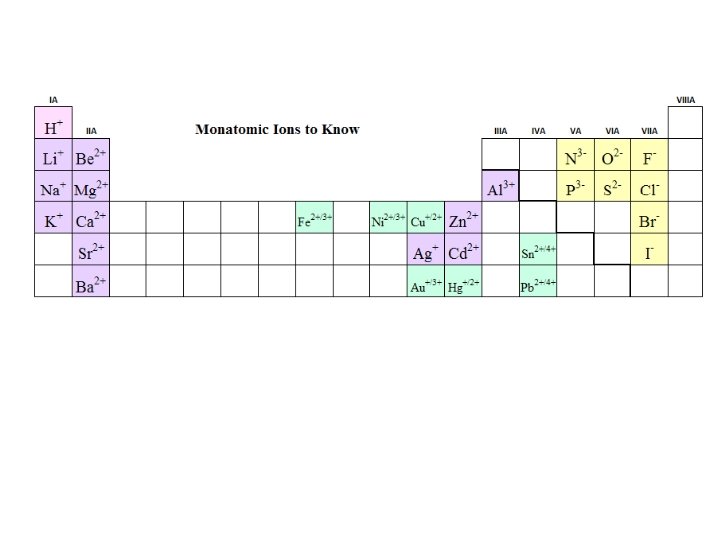
Ion Formation and Electron Dot Symbols: Na + N K + F Mg + O Al + S
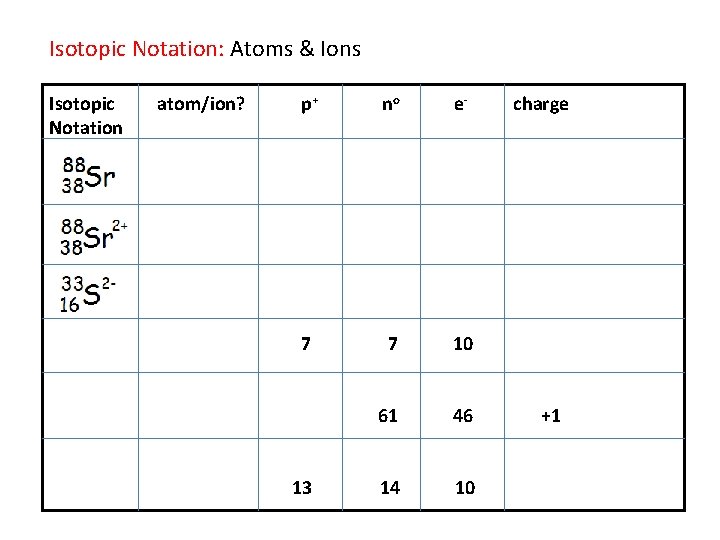
Isotopic Notation: Atoms & Ions Isotopic Notation atom/ion? p+ no e- 7 7 10 61 46 14 10 13 charge +1
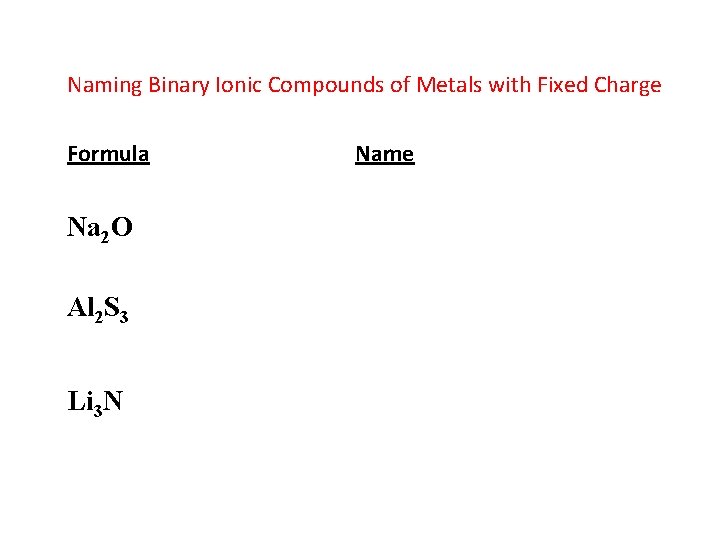
Naming Binary Ionic Compounds of Metals with Fixed Charge Formula Na 2 O Al 2 S 3 Li 3 N Name
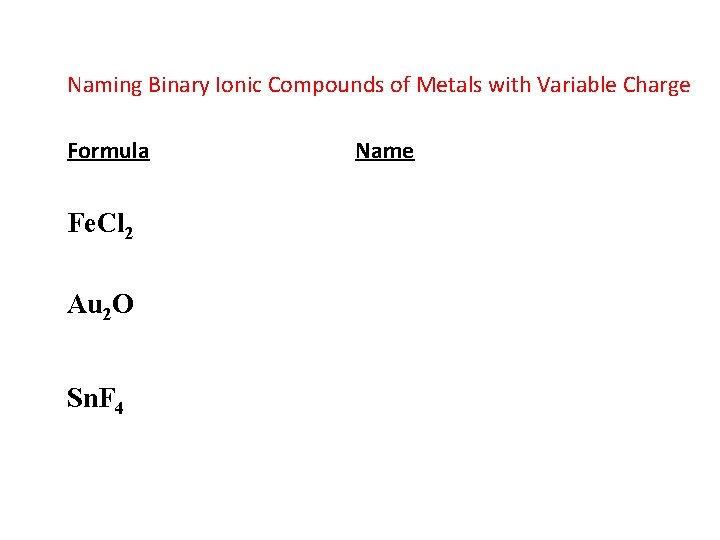
Naming Binary Ionic Compounds of Metals with Variable Charge Formula Fe. Cl 2 Au 2 O Sn. F 4 Name
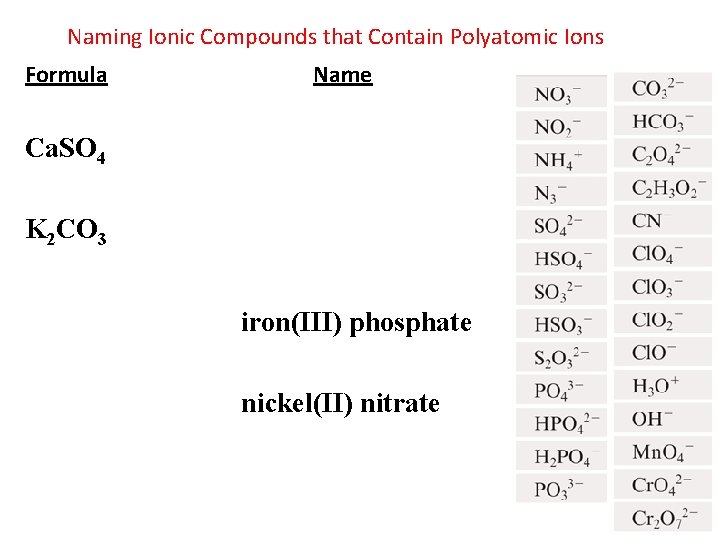
Naming Ionic Compounds that Contain Polyatomic Ions Formula Name Ca. SO 4 K 2 CO 3 iron(III) phosphate nickel(II) nitrate
- Slides: 13