CHAPTER 4 CARBON AND THE MOLECULAR DIVERSITY OF

CHAPTER 4 CARBON AND THE MOLECULAR DIVERSITY OF LIFE 1
Overview: Carbon—The Backbone of Life v carbon-based (organic). v v v 2
Concept 4. 1: Organic chemistry is the study of carbon compounds A. Organic chemistry 3
Concept 4. 2: Carbon atoms can form diverse molecules by bonding to four other atoms v v 4
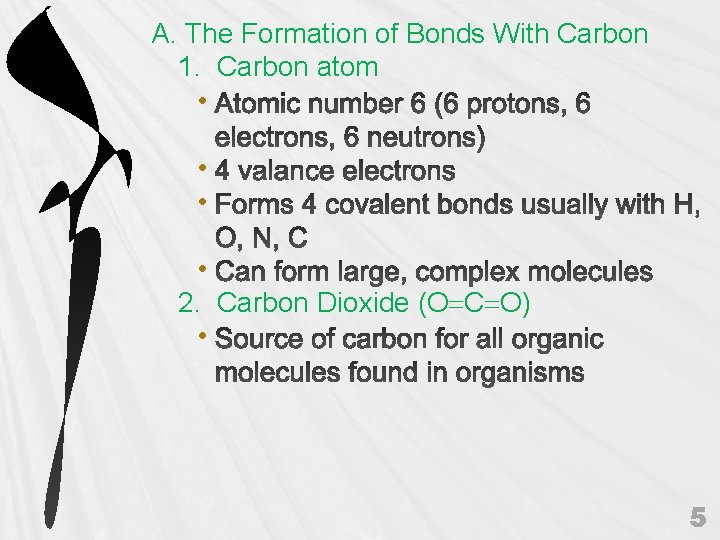
A. The Formation of Bonds With Carbon 1. Carbon atom • • 2. Carbon Dioxide (O C O) • 5
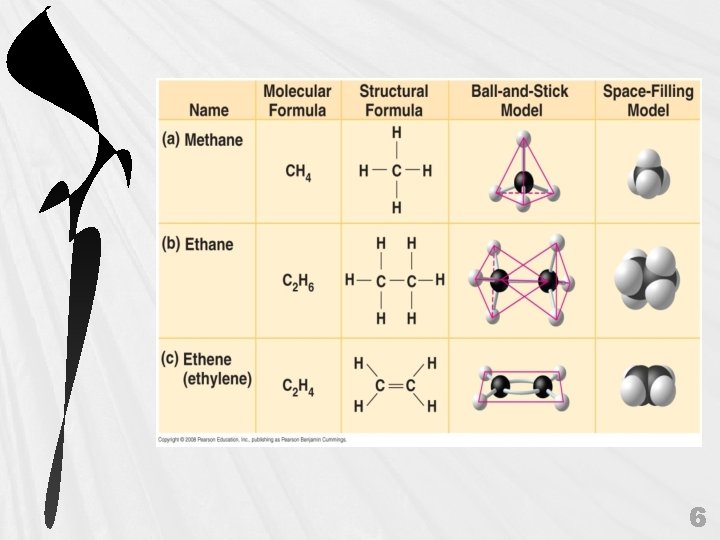
6
B. Molecular Diversity Arising from Carbon Skeleton Variation • • 7
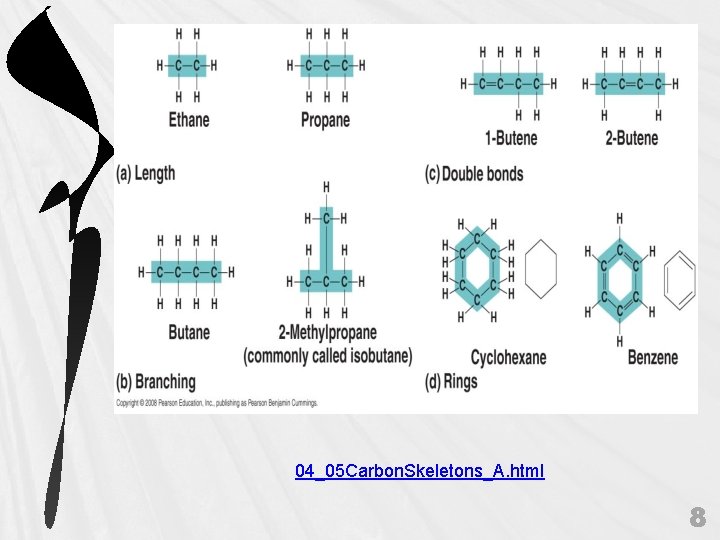
04_05 Carbon. Skeletons_A. html 8
C. Hydrocarbons 1. Hydrocarbons 2. 3. 4. hydrophobic 9
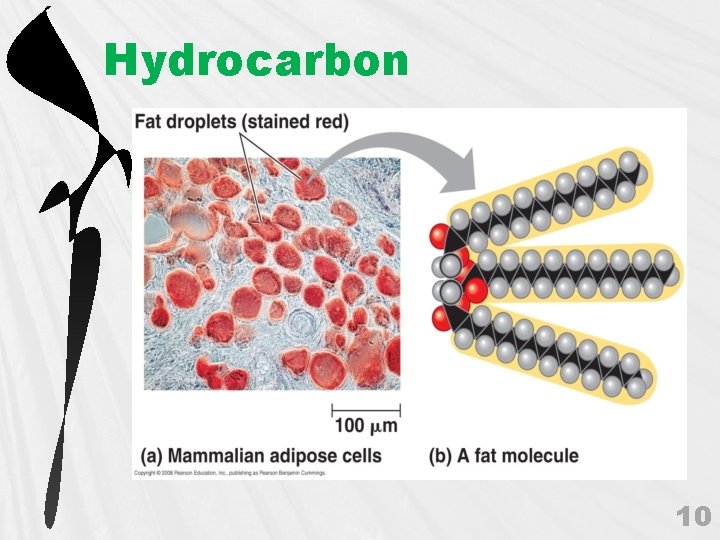
Hydrocarbon 10
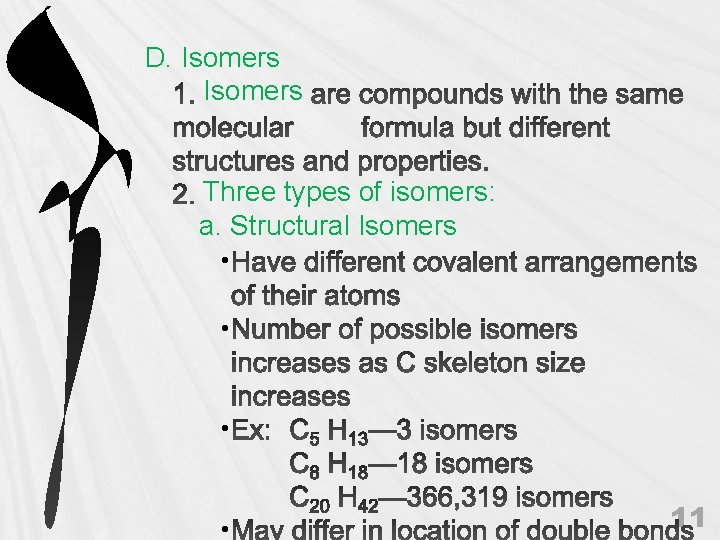
D. Isomers Three types of isomers: a. Structural Isomers • • 11
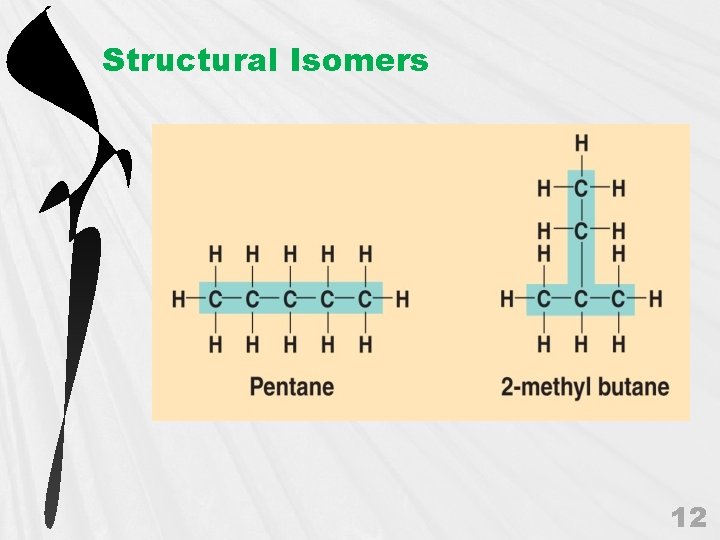
Structural Isomers 12
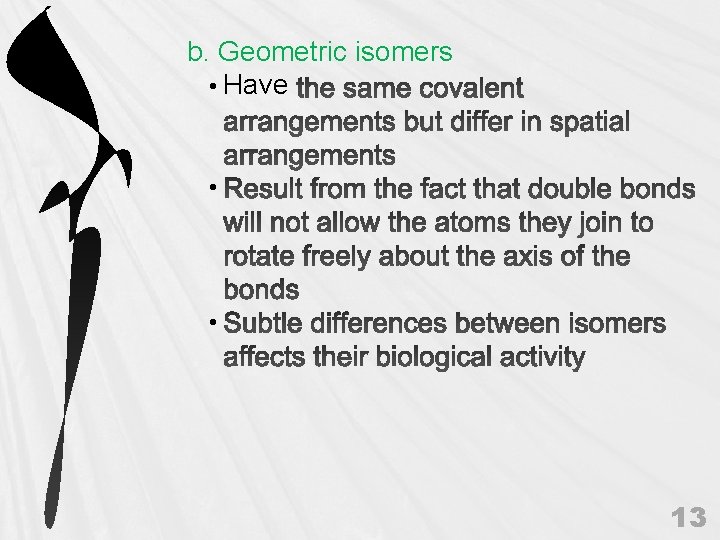
b. Geometric isomers • Have • • 13

Geometric Isomers 14
c. Enantiomers • • • 04_07 Isomers_A. html 15
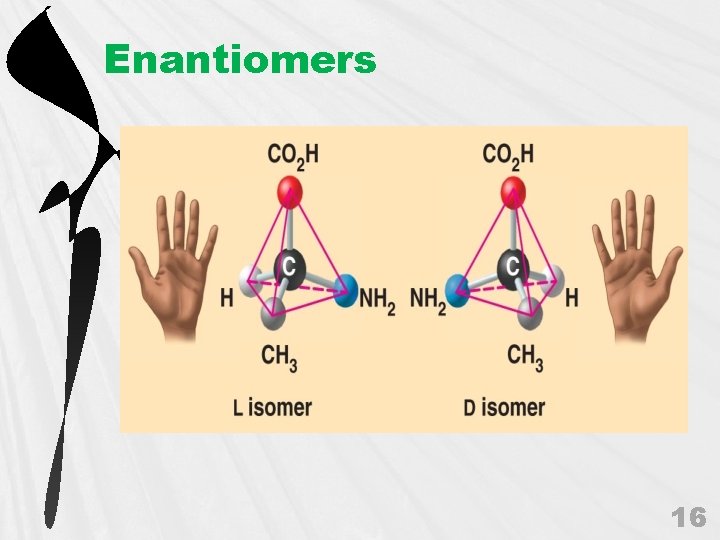
Enantiomers 16
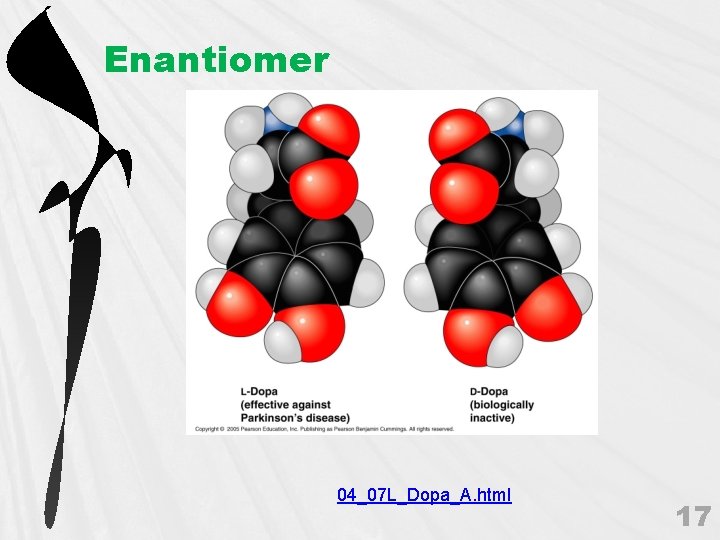
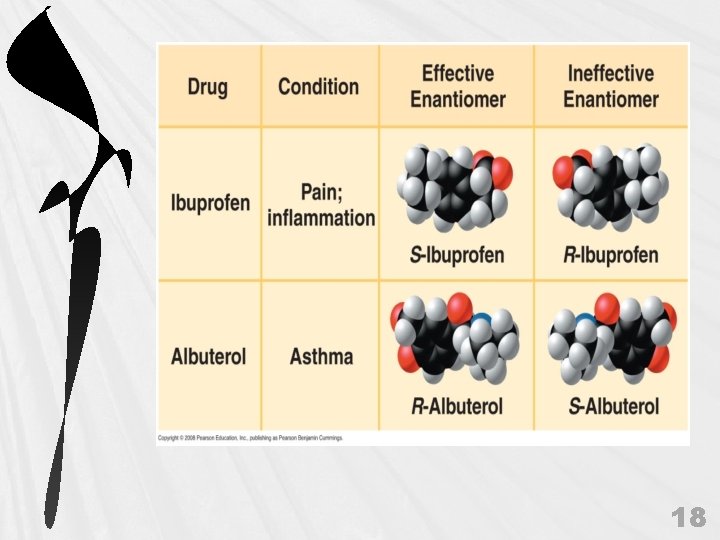
Enantiomer 04_07 L_Dopa_A. html 17
18
Concept 4. 3: A small number of chemical groups are key to the functioning of biological molecules v v 19
A. The Chemical Groups Most Important in the Process of Life 1. Functional groups 20
21
Hydroxyl group Carbonyl group Carboxyl group Amino group Sulfhydryl group Phosphate group Methyl group 22
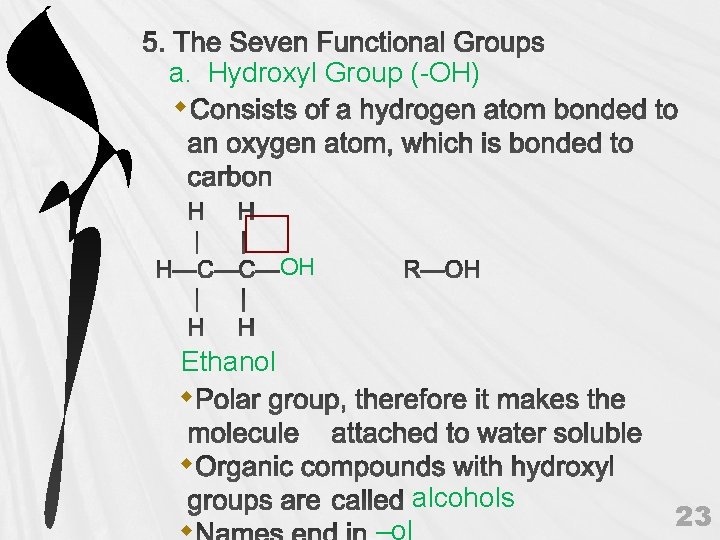
a. Hydroxyl Group (-OH) OH Ethanol alcohols 23
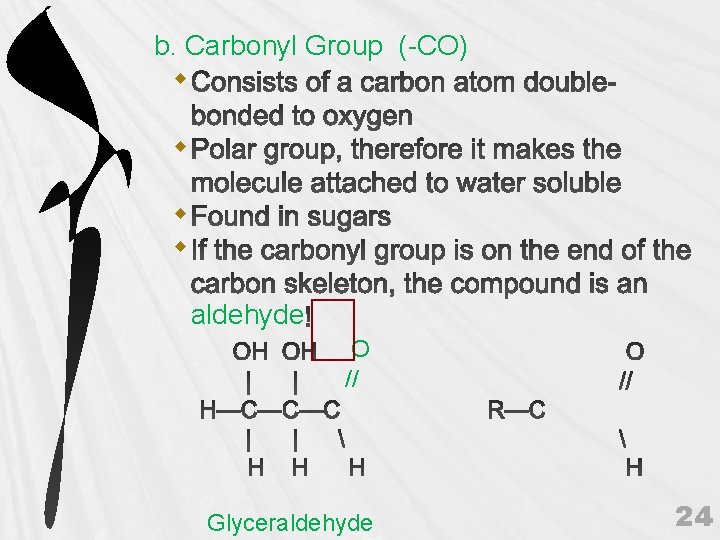
b. Carbonyl Group (-CO) aldehyde O // Glyceraldehyde 24
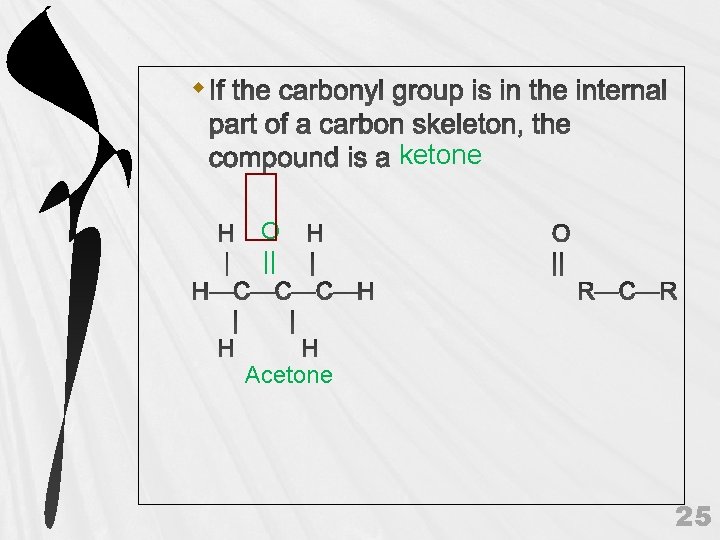
ketone O || Acetone 25
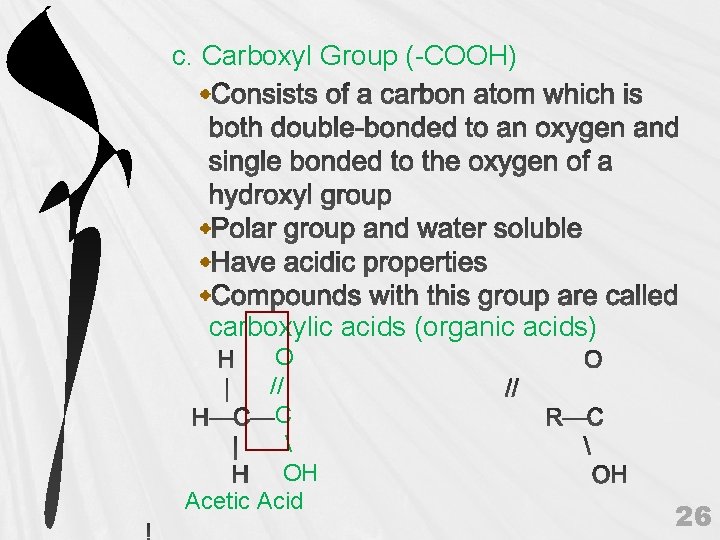
c. Carboxyl Group (-COOH) carboxylic acids (organic acids) O // C OH Acetic Acid 26
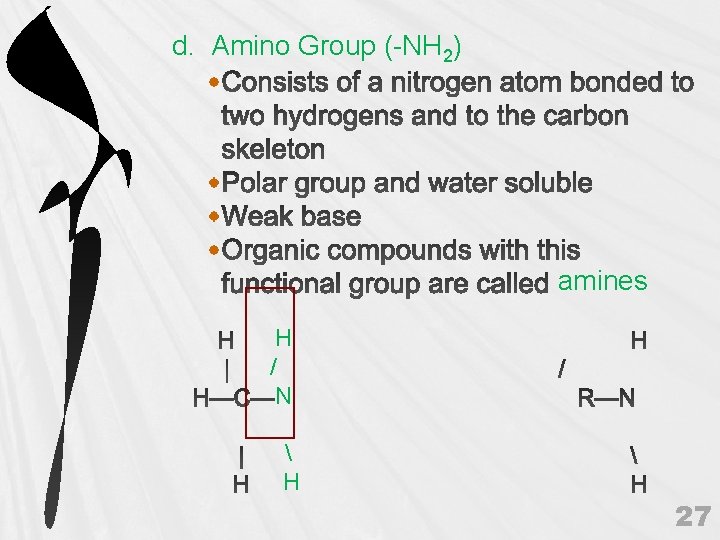
d. Amino Group (-NH 2) amines H / N H 27
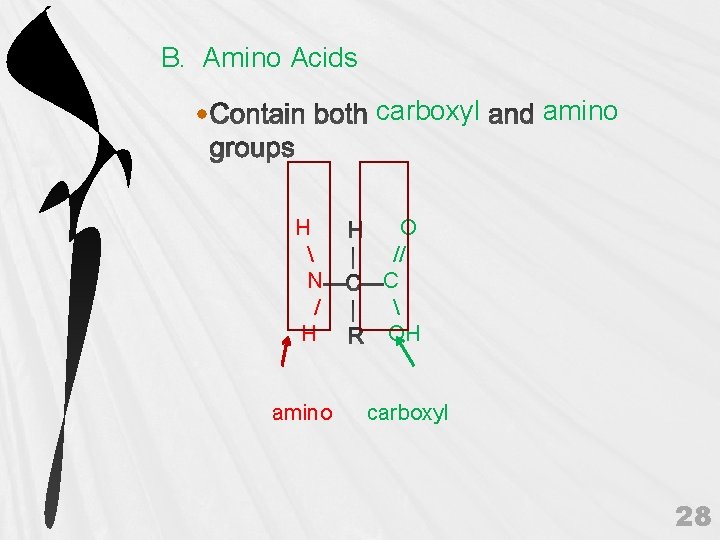
B. Amino Acids carboxyl H N / H amino O // C OH carboxyl 28
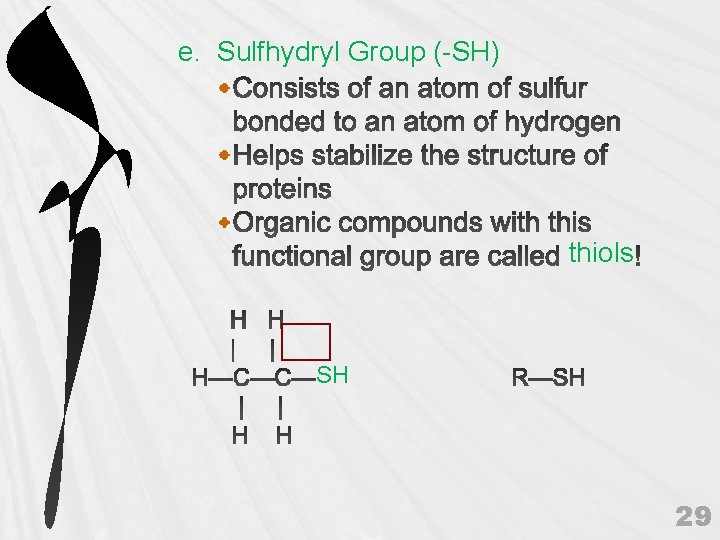
e. Sulfhydryl Group (-SH) thiols SH 29
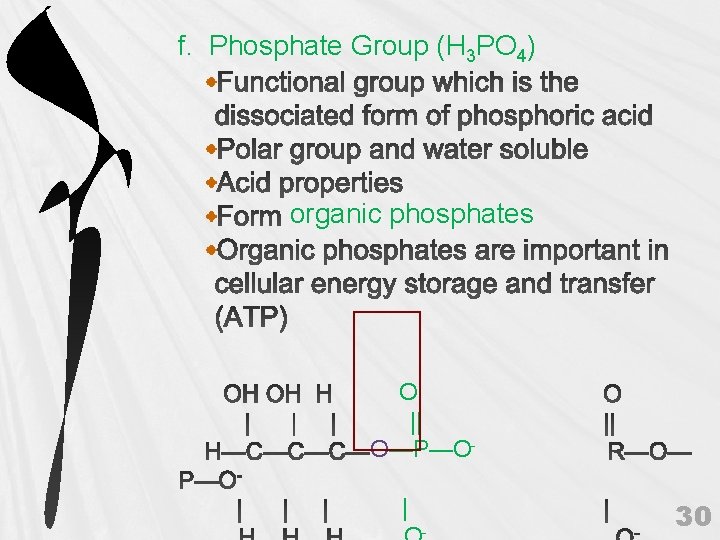
f. Phosphate Group (H 3 PO 4) organic phosphates O || O—P—O| - 30
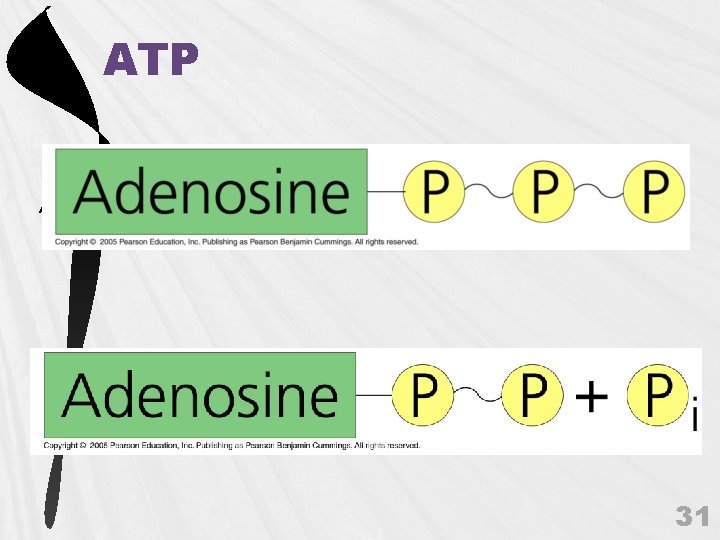
ATP 31
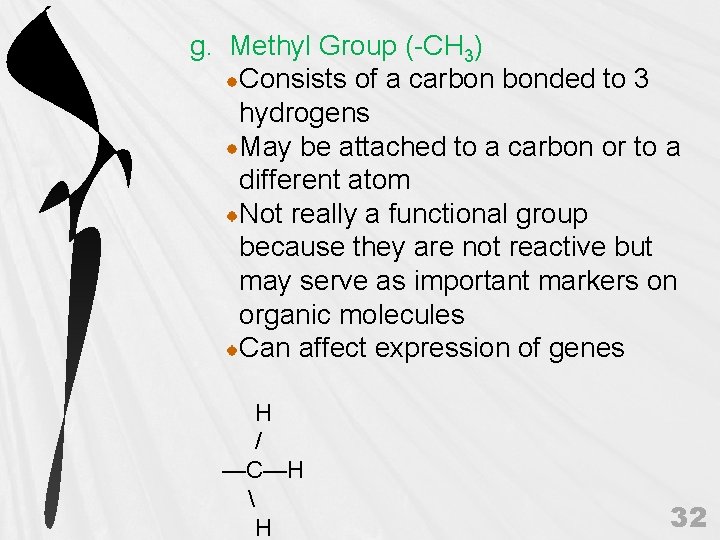
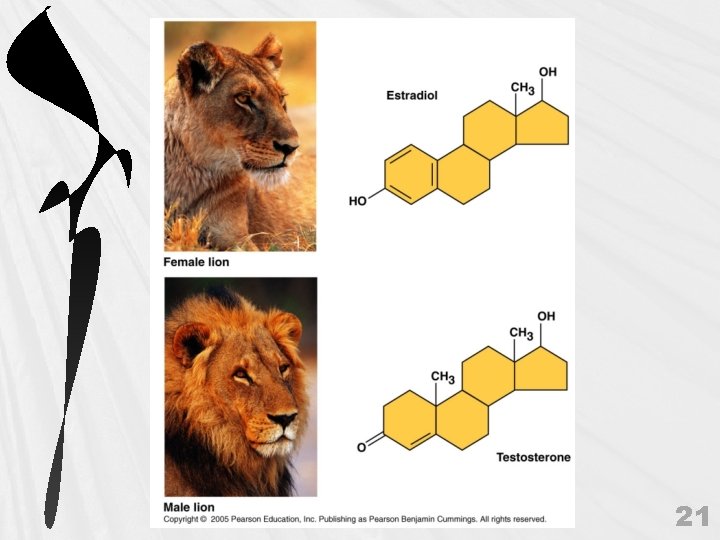
g. Methyl Group (-CH 3) Consists of a carbon bonded to 3 hydrogens May be attached to a carbon or to a different atom Not really a functional group because they are not reactive but may serve as important markers on organic molecules Can affect expression of genes H / —C—H H 32
- Slides: 32