CHAPTER 4 Atomic Structure The Rutherford Geiger and



- Slides: 17

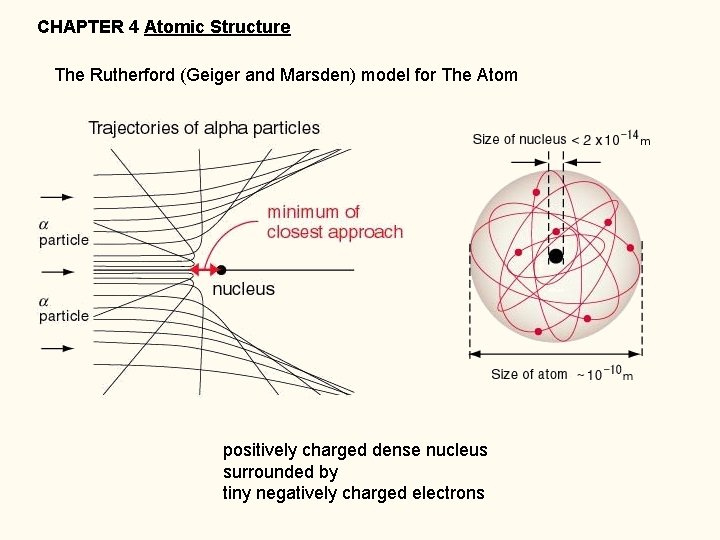
CHAPTER 4 Atomic Structure The Rutherford (Geiger and Marsden) model for The Atom positively charged dense nucleus surrounded by tiny negatively charged electrons

The Rutherford (Geiger and Marsden) model for The Atom positively charged dense nucleus radius __________ 3 x 10 -15 m (3 x 10 -13 cm) mass______ 2. 0 x 10 -23 g density ___________ 2 x 1014 g/cm 3 (water density 1 g/cm 3) 2 x 1014 g/cm 3 if all the cars in the world were smashed into a thimble surrounded by tiny negatively charged electrons in a VAST space… _____________ 10, 000 bigger than the nucleus http: //obeattie. github. io/gmaps-radius/? radius. Input=100&unit. Selector=mi&lat=46. 643088&lng=66. 163691&z=5&u=mi&r=500

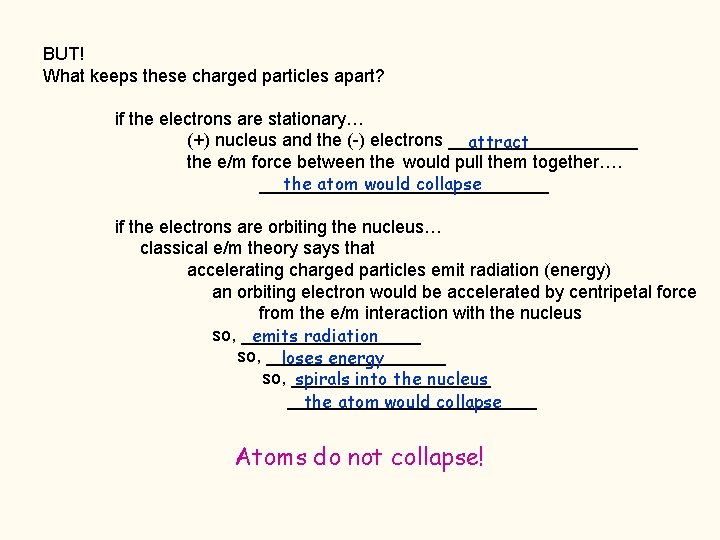
BUT! What keeps these charged particles apart? if the electrons are stationary… (+) nucleus and the (-) electrons __________ attract the e/m force between the would pull them together…. _______________ the atom would collapse if the electrons are orbiting the nucleus… classical e/m theory says that accelerating charged particles emit radiation (energy) an orbiting electron would be accelerated by centripetal force from the e/m interaction with the nucleus so, _________ emits radiation so, _________ loses energy so, __________ spirals into the nucleus _____________ the atom would collapse Atoms do not collapse!

BUT also… The Rutherford model doesn’t explain the behavior of matter… specifically, there is nothing in the model to account for: ______________ - bonding between atoms ______________ - reactivity ______________ - physical properties AND ___________________ - the interaction of light with matter (color)

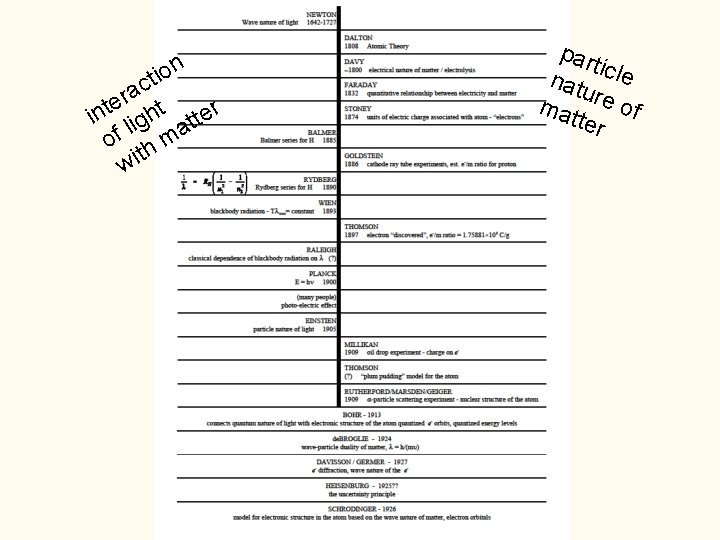
on i t c a r e ht t r n e i lig t t a f o hm t wi par ticle natu mat re of ter

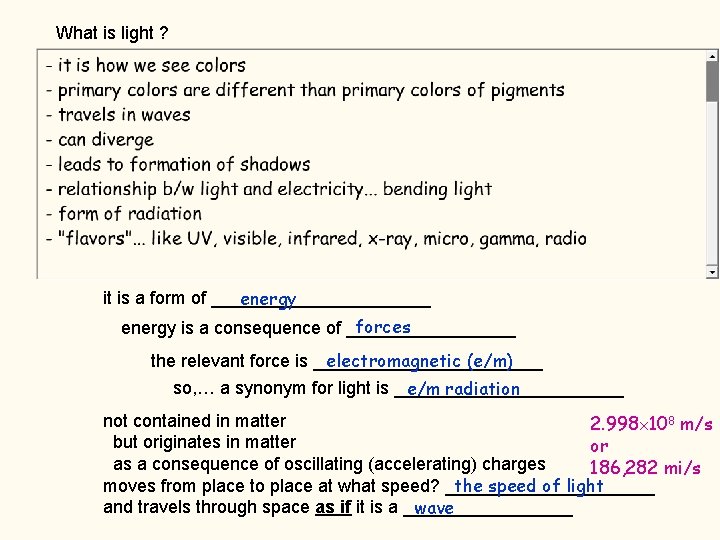
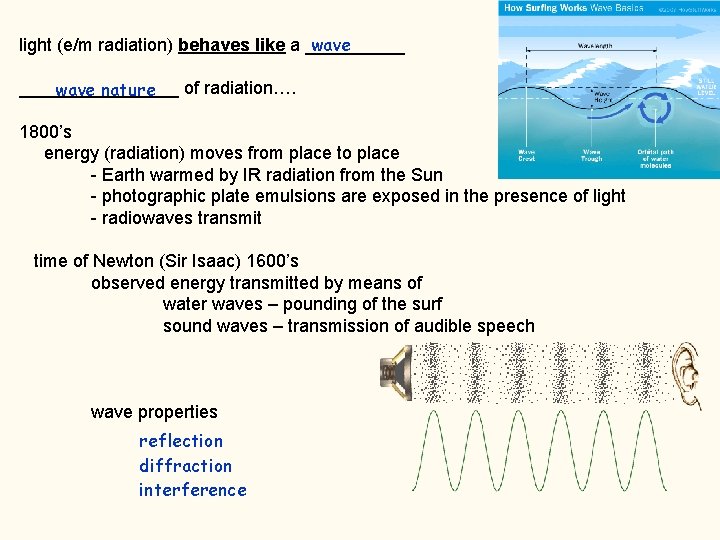
What is light ? it is a form of ___________ energy forces energy is a consequence of _________ the relevant force is ____________ electromagnetic (e/m) so, … a synonym for light is ____________ e/m radiation not contained in matter 2. 998 108 m/s but originates in matter or as a consequence of oscillating (accelerating) charges 186, 282 mi/s moves from place to place at what speed? ___________ the speed of light and travels through space as if it is a _________ wave

wave light (e/m radiation) behaves like a ________________ of radiation…. wave nature 1800’s energy (radiation) moves from place to place - Earth warmed by IR radiation from the Sun - photographic plate emulsions are exposed in the presence of light - radiowaves transmit time of Newton (Sir Isaac) 1600’s observed energy transmitted by means of water waves – pounding of the surf sound waves – transmission of audible speech wave properties reflection diffraction interference

link to reflection_diffraction_interference links http: //www. youtube. com/watch? v=J_xd 9 h. UZ 2 AY http: //www. youtube. com/watch? v=fja. PGk. OX-wo powerpoint interference diffraction links http: //www. youtube. com/watch? v=BH 0 Nf. VUTWG 4 reflection links

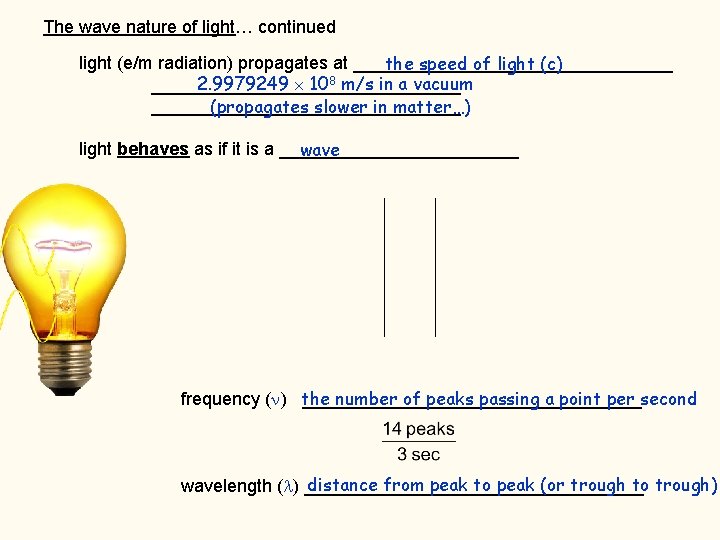
The wave nature of light… continued light (e/m radiation) propagates at ________________ the speed of light (c) 2. 9979249 108 m/s in a vacuum _______________________________ (propagates slower in matter…) light behaves as if it is a ____________ wave number of peaks passing a point per second frequency ( ) the _________________ distance from peak to peak (or trough to trough) wavelength ( ) _________________

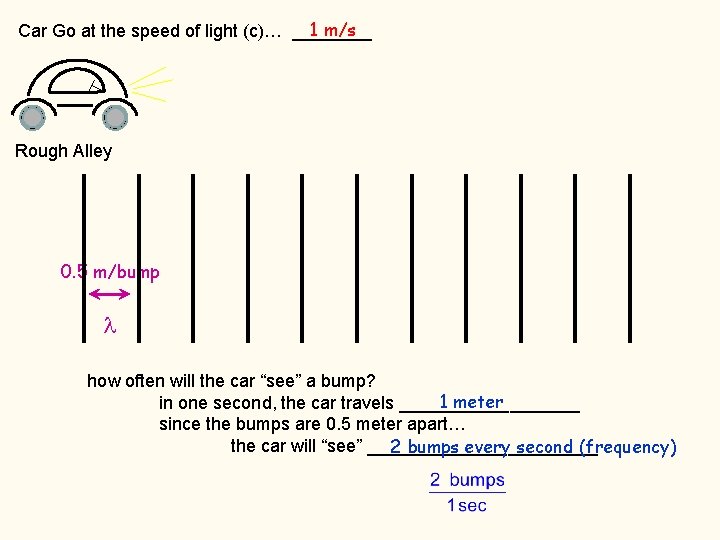
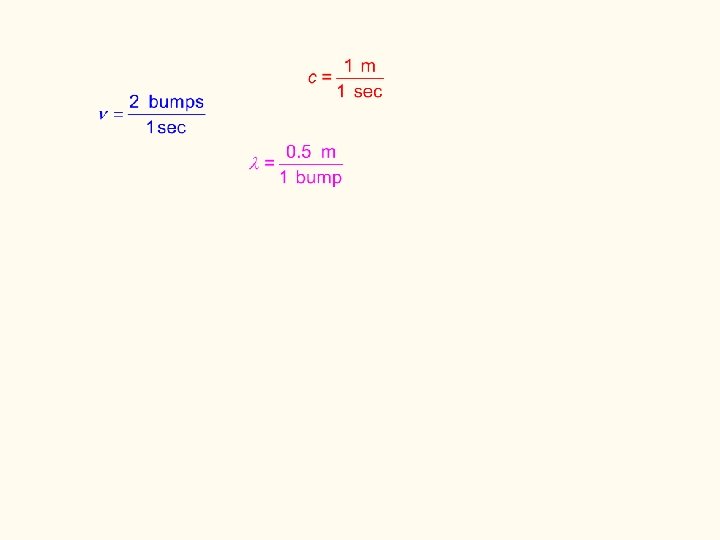
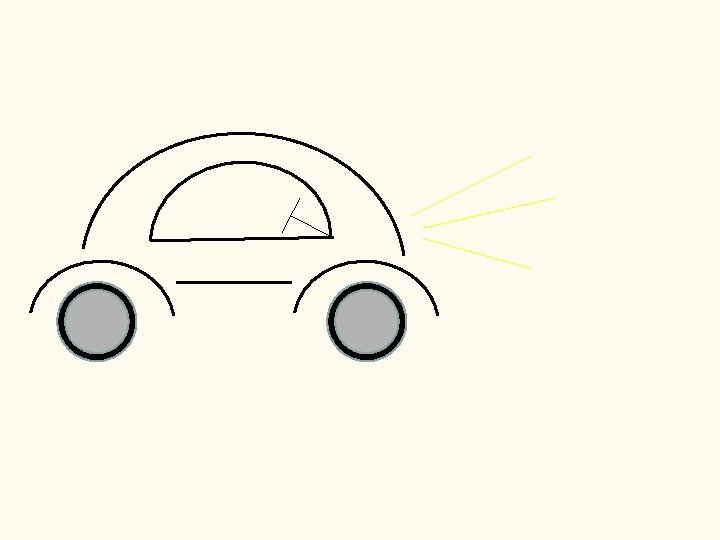
build the relationship between c, , and The Ronabile 1 m/s Car Go at the speed of light (c)… ____

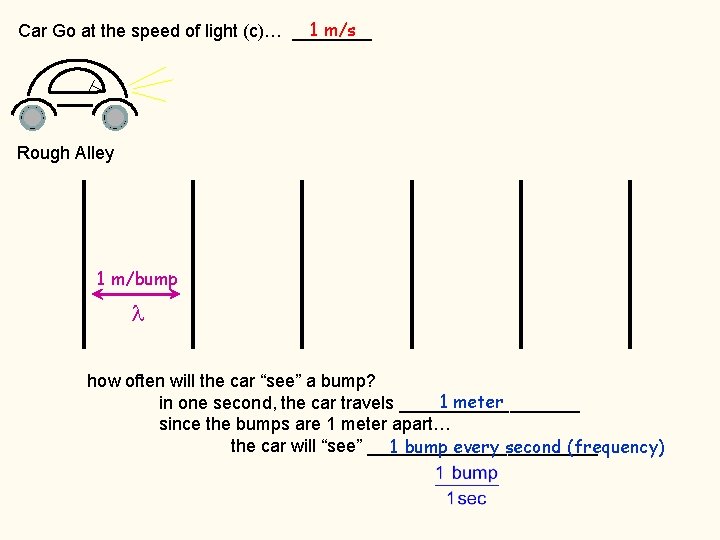
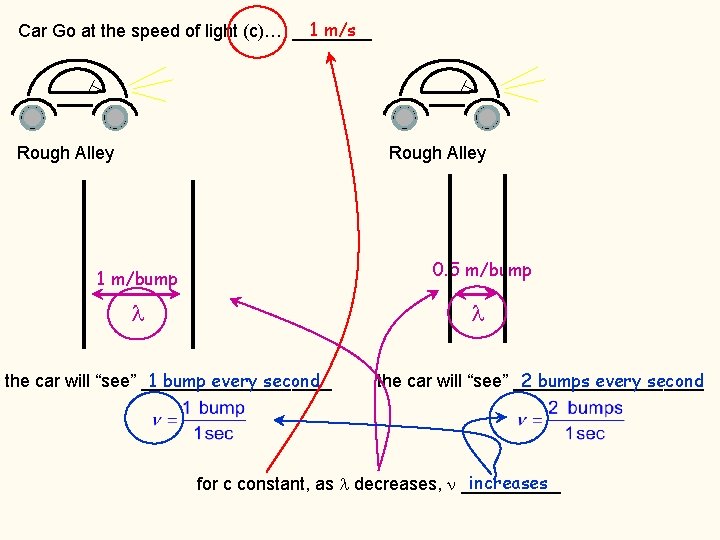
1 m/s Car Go at the speed of light (c)… ____ Rough Alley 1 m/bump how often will the car “see” a bump? 1 meter in one second, the car travels _________ since the bumps are 1 meter apart… the car will “see” ____________ 1 bump every second (frequency)

1 m/s Car Go at the speed of light (c)… ____ Rough Alley 0. 5 m/bump how often will the car “see” a bump? 1 meter in one second, the car travels _________ since the bumps are 0. 5 meter apart… the car will “see” ____________ 2 bumps every second (frequency)

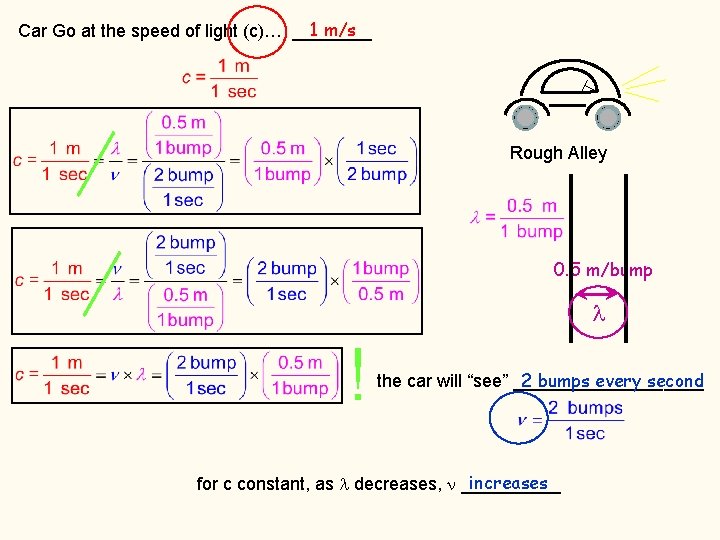
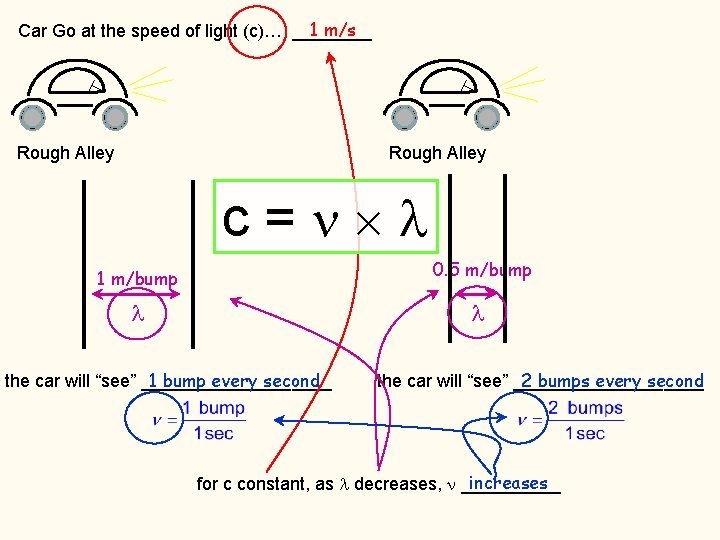
1 m/s Car Go at the speed of light (c)… ____ Rough Alley 0. 5 m/bump 1 m/bump 1 bump every second the car will “see” __________ 2 bumps every second the car will “see” __________ increases for c constant, as decreases, _____

1 m/s Car Go at the speed of light (c)… ____ Rough Alley 0. 5 m/bump ! 2 bumps every second the car will “see” __________ increases for c constant, as decreases, _____

1 m/s Car Go at the speed of light (c)… ____ Rough Alley c= 0. 5 m/bump 1 m/bump 1 bump every second the car will “see” __________ 2 bumps every second the car will “see” __________ increases for c constant, as decreases, _____

