Chapter 4 Arrangement of Electrons in Atoms Chapter








- Slides: 22

Chapter 4 Arrangement of Electrons in Atoms

Chapter 4 -1 • The Development of a New Atomic Model! • Review Waves: • Crest, trough, wavelength, amplitude, frequency • Electromagnetic Spectrum/ Visible light Spectrum

Properties of light • The Emission of light is fundamentally related to the behavior of electrons. • In the early twentieth century a new atomic model evolved as a result of investigations into the absorption and emission of light by matter.

Properties of Light • Visible light is a type of energy in the electromagnetic spectrum. • The Electromagnetic Spectrumelectromagnetic radiation is a form of energy that exhibits wavelike behavior as it travels through space.

Speed of Light Formula • C= wavelength x frequency lamda nu • C= Speed of Light = 3. 00 x 10 meters/ sec 8

The Photoelectric Effect • The photoelectric effect refers to the emission of electrons from a metal when light shines on the metal. – Not all types of light can cause the photoelectric effect – Light had to have a certain frequency or “energy” to cause this effect.

The Particle Description of Light • Max Planck 1900 believed hot object emitted light not as continuous energy but in specific packets of energy called Quanta. • Quanta is the minimum quantity of energy that can be lost or gained by an atom.

E= hv • Energy of a photon of light is equal to Planck’s constant times frequency. • Planck’s Constant is 6. 626 x 10 -34 joules*sec

Albert Einstein – In 1905 Einstein expanded Planck’s theory to include the radical idea that electromagnetic radiation has a dual wave-particle nature. – Photon is a particle of electro-magnetic radiation having zero mass and carrying a quanta of energy

The Hydrogen-Atom Line-Emission Spectrum • Ground State- lowest energy state of an atom • Excited State- state in which an atom has a higher potential energy than in its ground state. • Line Emission Spectrum= fingerprint of an element.

Bohr Model of H Atom • 1913 Danish physicist Niels Bohr According to the Model, the electrons can circle the nucleus only in allowed pathways , or orbits. • He correctly calculate the absorption and emission energies for Hydrogen.

Chapter 4 Section 2 • The quantum Model of the Atom – In the early twentieth century, electrons were determined to have a dual wave-particle energy.

The Heisenberg Uncertainty Principle • The Heisenberg uncertainty principle states that it is impossible to determine simultaneously the position and velocity of an electron or any other particle.

The Schrodinger Wave Equation • 1926 Erwin Schrodinger (Austrian) physicist developed a incredible complicated “wave” equation. It confirmed mathematically that electron behave like waves and can only produce certain quantized energies.

Quantum Theory • Based on Bohr’s Model, Heisenberg’ s Uncertainty Principle and the Schrodinger Wave Equation • Quantum Theory was born! • Quantum Theory describes mathematically the wave properties of electrons.

Atomic Orbitals and Quantum Numbers • Principal Quantum number (n) • Angular Momentum Quantum number (l) • Magnetic Quantum number (m) • Spin Quantum number (s)

Definitions of Quantum Numbers • n=indicates the main energy level occupied by the electron • l=indicates the shape of the orbital • m=indicates the orientation of an orbital around the nucleus • s=indicates the spin

Section 4 -3 Electron Configurations • Electron Configuration is a user friendly application of Quantum Numbers! • Aufbau Principle: an electron will occupies the lowest- energy orbital that can receive it. A. K. A. The Diagonal Rule ( p. 116)

Electron Con. Fig. • Examples: page 116 sequence! • Always the same. • Ex: Nitrogen 7 electrons • 1 s 22 p 3 Ex: Sodium 11 electrons 1 s 22 p 63 s 1

More Electron Config. • Pauli’s exclusion principle: no two electrons in the same atom can have the same set of four quantum #’s • Hund’s Rule: electrons in a partially filled sub-level will spread out in the orbitals and maintain same spin before doubling up!

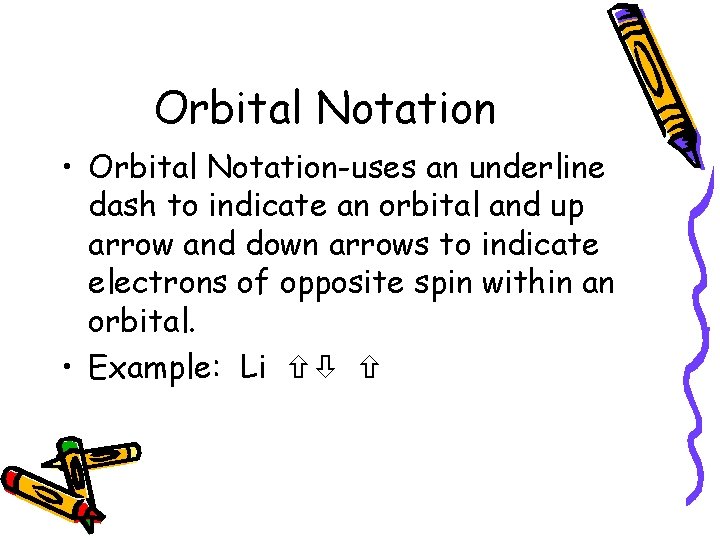
Orbital Notation • Orbital Notation-uses an underline dash to indicate an orbital and up arrow and down arrows to indicate electrons of opposite spin within an orbital. • Example: Li

Nobel Gas Con. Fig. • Noble Gas Configuration: • Helpful abbreviation of electron con fig for large atoms with many electrons. • Examlp: Al 13 Electrons • [Ne] 3 s 23 p 1