CHAPTER 4 ARRANGEMENT OF ELECTRONS IN ATOMS 4











- Slides: 24

CHAPTER 4: ARRANGEMENT OF ELECTRONS IN ATOMS

4. 1 THE DEVELOPMENT OF A Essential Questions: NEW ATOMIC MODEL 1. What is the mathematical relationship between the speed, wavelength and frequency of electromagnetic radiation? 2. What is the dual wave-particle nature of light? 3. What relationship does the line emission spectrum of Hydrogen have with the development of the atomic model? 4. Describe the Bohr model of the hydrogen atom.

I. PROPERTIES OF LIGHT

A. Electromagnetic Radiation (EM Radiation) 1. Many types of EM waves a. gamma rays b. x-rays c. ultraviolet light d. visible light e. infrared light f. microwave g. radio waves

EM radiation are forms of energy that move through space as waves. a. Move at speed of light, (abbreviated as c) 3. 00 x 108 m/s or (300, 000 km/s) Speed of the wave is NOT the same as wavelength or frequency!!!

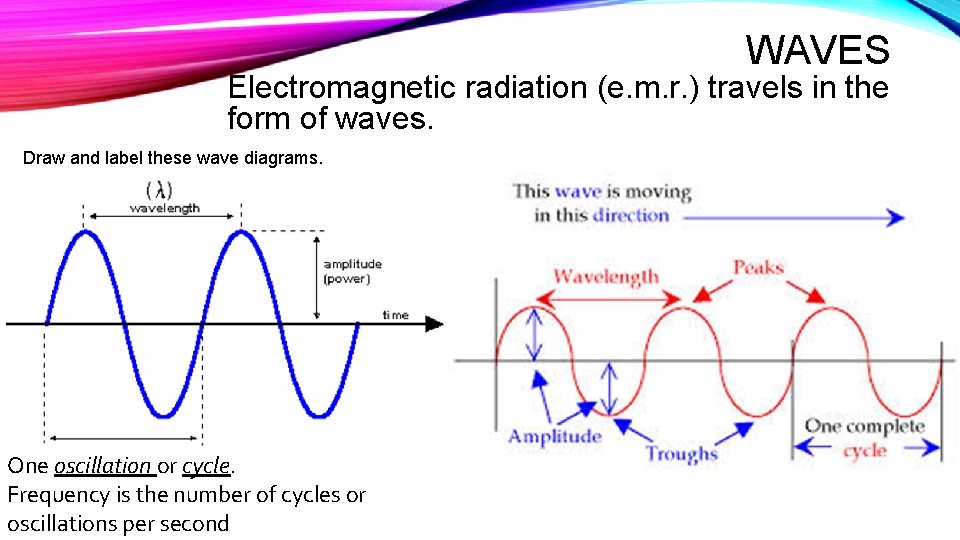
WAVES Electromagnetic radiation (e. m. r. ) travels in the form of waves. Draw and label these wave diagrams. One oscillation or cycle. Frequency is the number of cycles or oscillations per second

SPEED, FREQUENCY AND WAVELENGTH b. Speed is equal to the frequency times the wavelength c=νλ (1) Frequency (ν) is the number of waves passing a given point in one second. (2) Wavelength (λ) is the distance between peaks of adjacent waves. (3) The speed of light (c)is 300, 000 km/s.

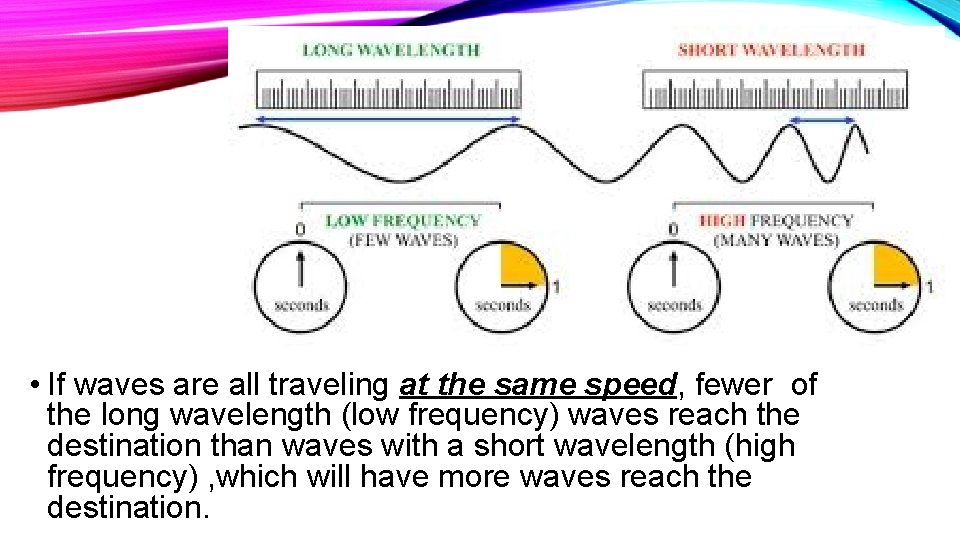
• If waves are all traveling at the same speed, fewer of the long wavelength (low frequency) waves reach the destination than waves with a short wavelength (high frequency) , which will have more waves reach the destination.

c. Speed of light is a constant, so ν λ is also a constant. ν and λ must be inversely proportional. When something is inversely proportional, one value gets larger while the other value gets smaller and vice versa.

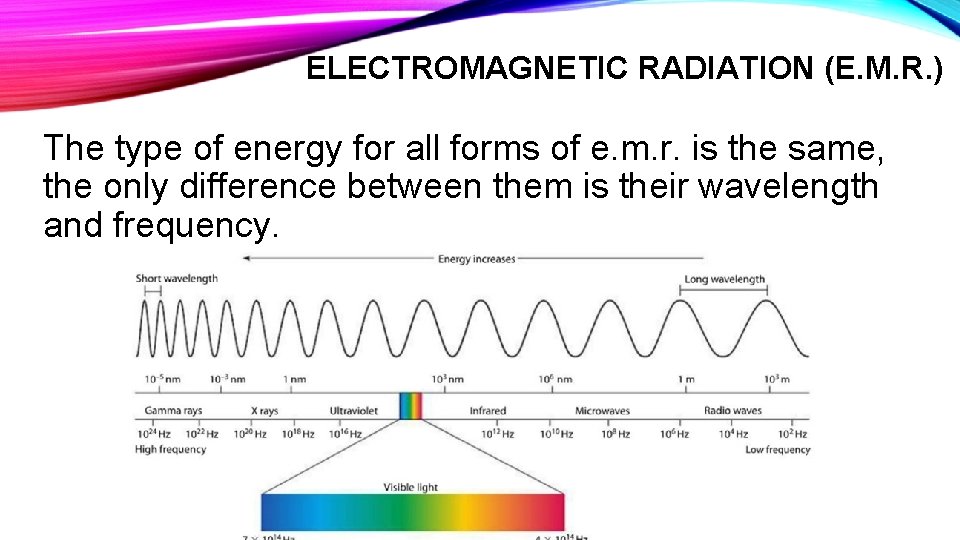
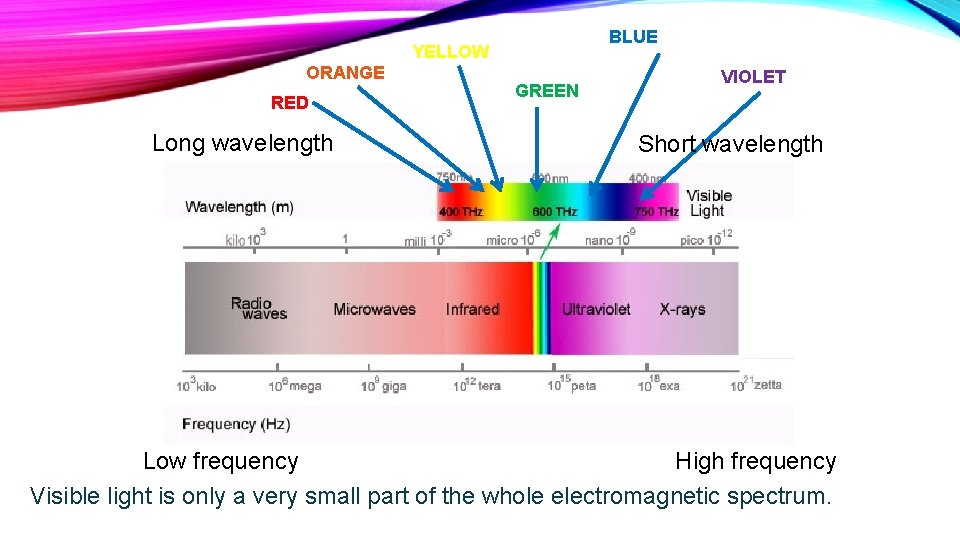
ELECTROMAGNETIC RADIATION (E. M. R. ) The type of energy for all forms of e. m. r. is the same, the only difference between them is their wavelength and frequency.

BLUE YELLOW ORANGE RED Long wavelength GREEN VIOLET Short wavelength Low frequency High frequency Visible light is only a very small part of the whole electromagnetic spectrum.

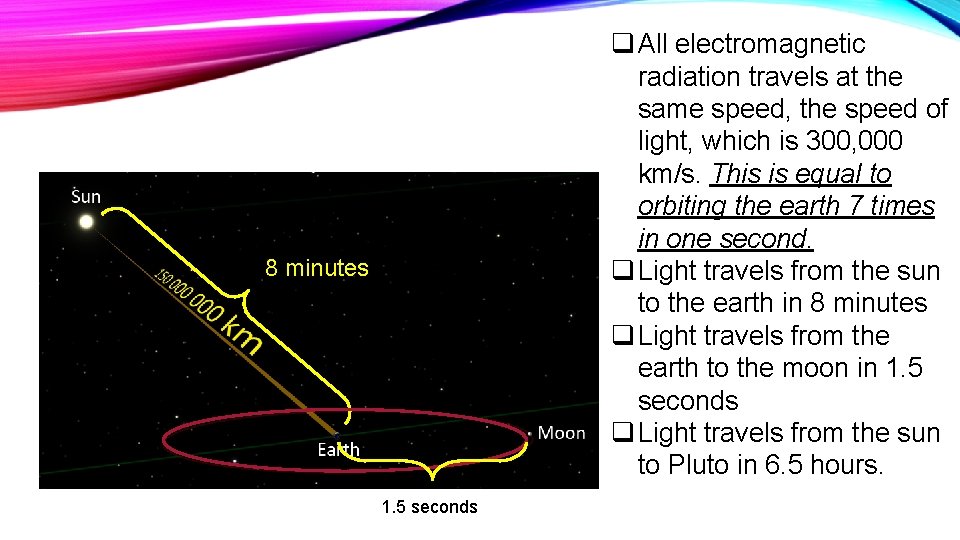
q All electromagnetic radiation travels at the same speed, the speed of light, which is 300, 000 km/s. This is equal to orbiting the earth 7 times in one second. q Light travels from the sun to the earth in 8 minutes q Light travels from the earth to the moon in 1. 5 seconds q Light travels from the sun to Pluto in 6. 5 hours. 8 minutes 1. 5 seconds

B. LIGHT AND ENERGY (THE PHOTOELECTRIC EFFECT) 1. Radiant energy is transferred in units of energy called photons. a. The units are called quanta-(plural of quantum) b. A photon is a particle of energy having a rest mass of zero and carrying a quantum of energy. c. A quantum is the minimum amount of energy that can be lost or gained by an atom.

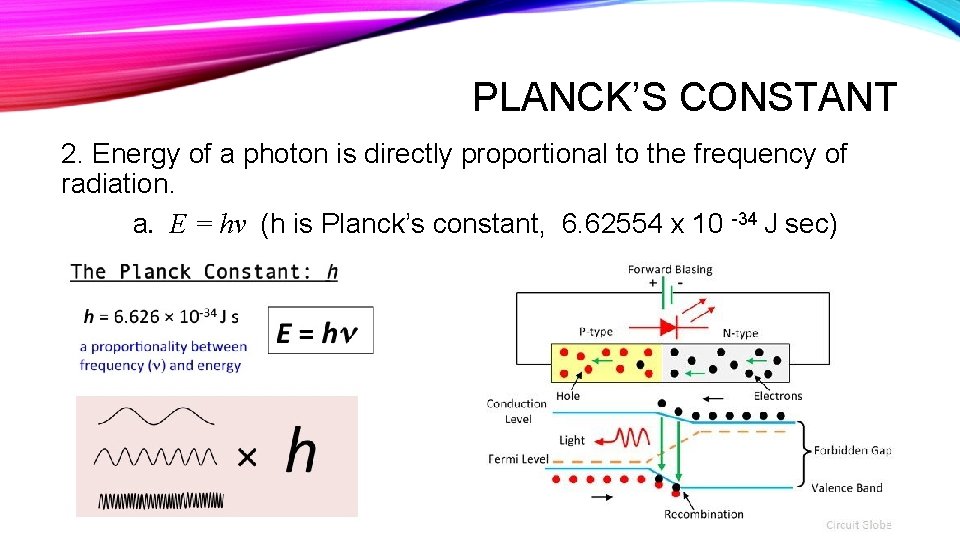
PLANCK’S CONSTANT 2. Energy of a photon is directly proportional to the frequency of radiation. a. E = hν (h is Planck’s constant, 6. 62554 x 10 -34 J sec)

II. ATOMIC SPECTRA

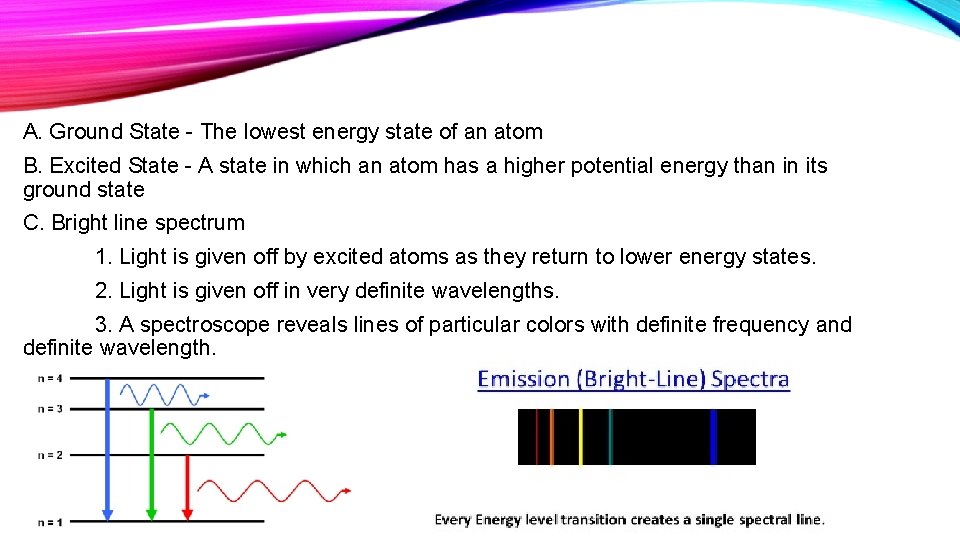
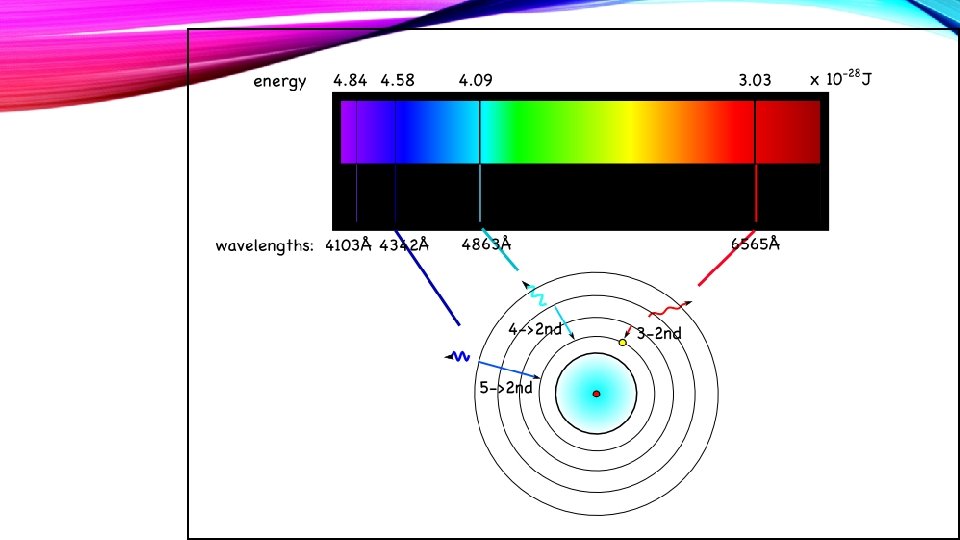
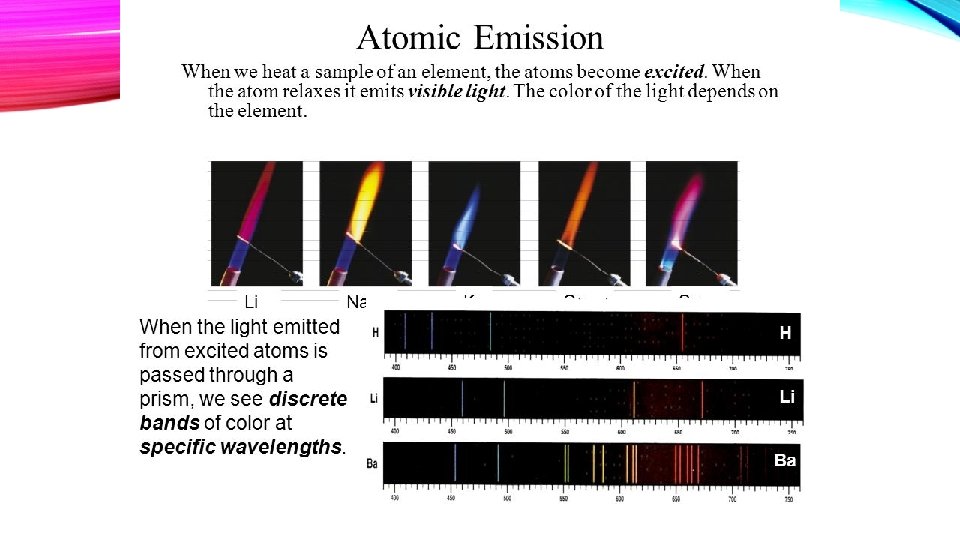
A. Ground State - The lowest energy state of an atom B. Excited State - A state in which an atom has a higher potential energy than in its ground state C. Bright line spectrum 1. Light is given off by excited atoms as they return to lower energy states. 2. Light is given off in very definite wavelengths. 3. A spectroscope reveals lines of particular colors with definite frequency and definite wavelength.



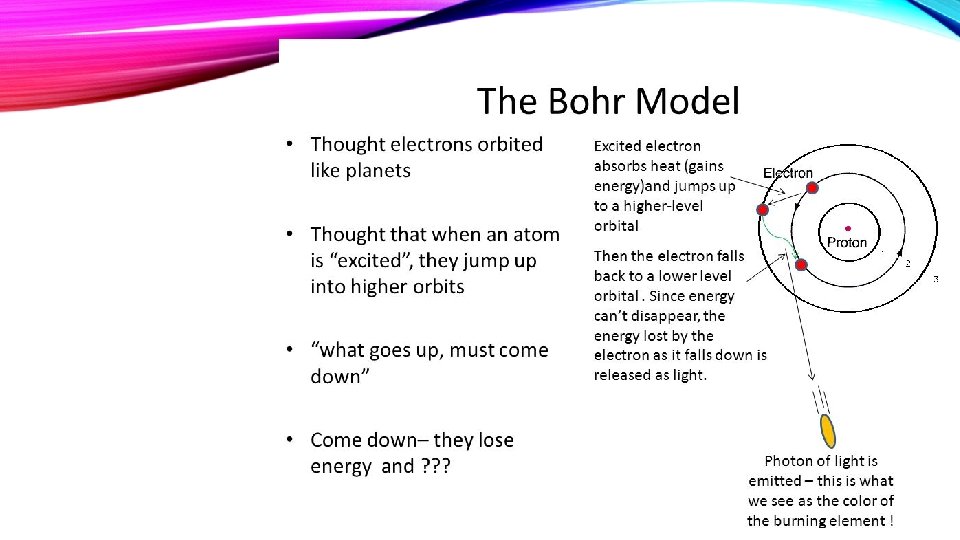
III. THE BOHR MODEL OF THE ATOM

A. Electron Orbits, or Energy Levels 1. Electrons can circle the nucleus only in allowed paths or orbits. 2. The energy of the electron is greater when it is in orbits farther from the nucleus. 3. The atom achieves the ground state when electrons occupy the closest possible positions around the nucleus. 4. Electromagnetic radiation is emitted when electrons move closer to the nucleus.


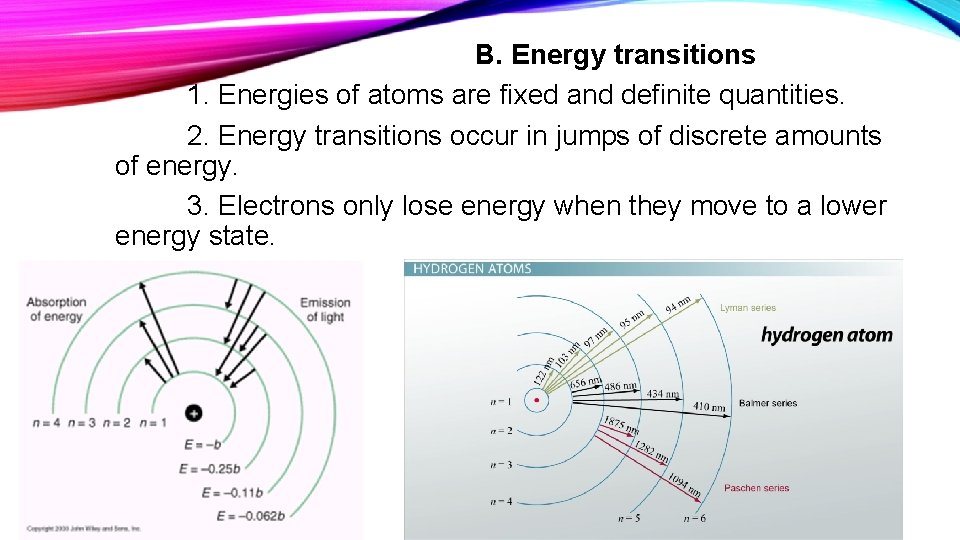
B. Energy transitions 1. Energies of atoms are fixed and definite quantities. 2. Energy transitions occur in jumps of discrete amounts of energy. 3. Electrons only lose energy when they move to a lower energy state.

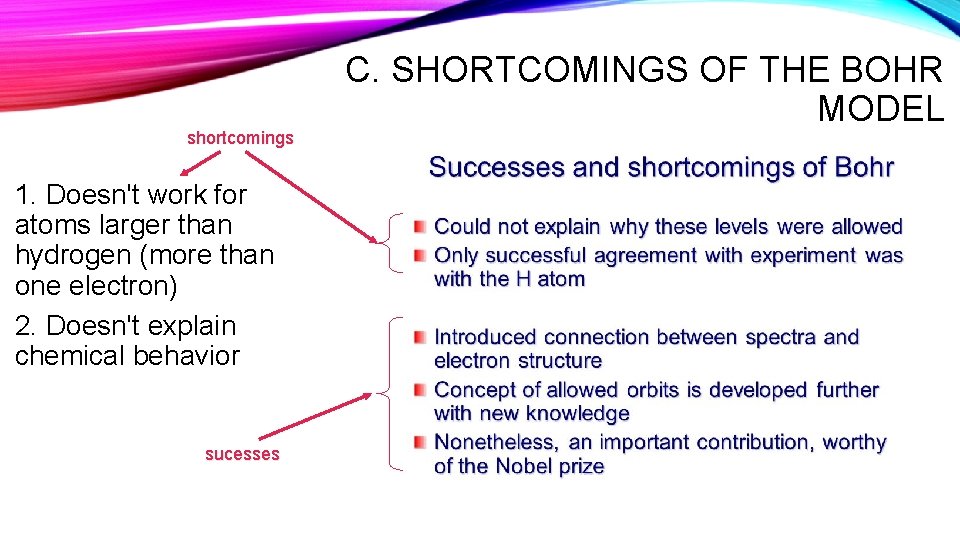
shortcomings 1. Doesn't work for atoms larger than hydrogen (more than one electron) 2. Doesn't explain chemical behavior sucesses C. SHORTCOMINGS OF THE BOHR MODEL

FINISH YOUR NOTES! • Reread the notes • Color Code • Add annotations • Write a summary • Write a response