Chapter 4 Arrangement of Electrons in an Atom











- Slides: 40

Chapter 4 Arrangement of Electrons in an Atom

4. 1 Refinements of the atomic model o Models of the atom so far: n n n Dalton – atoms are like little “bb’s” - then the electron gets discovered Thomson – atom is like a charged “bb” Rutherford - Gold foil experiment – hollow charged “bb” Bohr model of the atom (1913) – Neils Bohr – Danish Physicist The Bohr model of the atom comes from the idea that light is waves of energy http: //web. visionlearning. com/custom/chemistry/animations/CHE 1. 2 -an-atoms. shtml

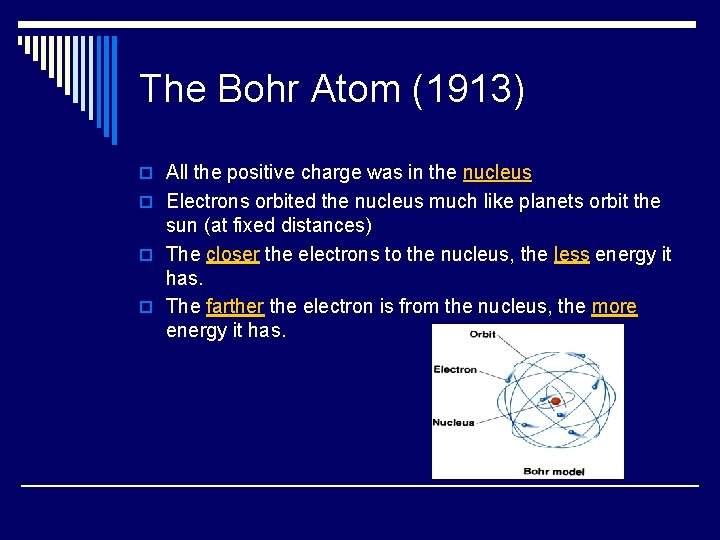
The Bohr Atom (1913) o All the positive charge was in the nucleus o Electrons orbited the nucleus much like planets orbit the sun (at fixed distances) o The closer the electrons to the nucleus, the less energy it has. o The farther the electron is from the nucleus, the more energy it has.

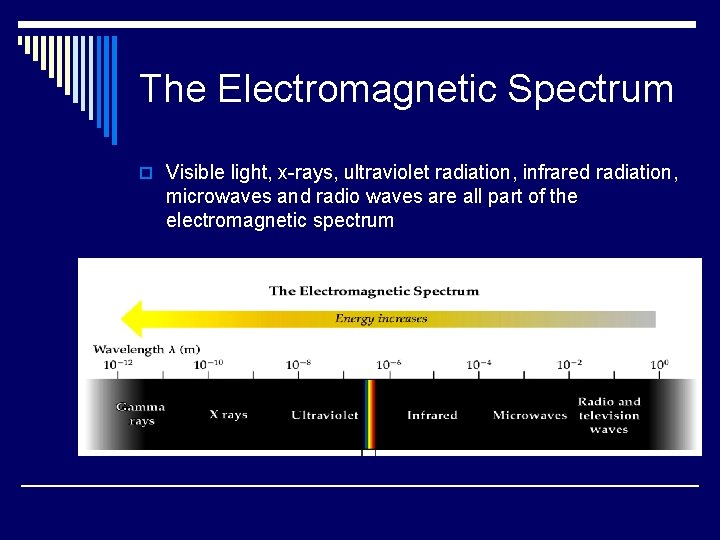
The Electromagnetic Spectrum o Visible light, x-rays, ultraviolet radiation, infrared radiation, microwaves and radio waves are all part of the electromagnetic spectrum

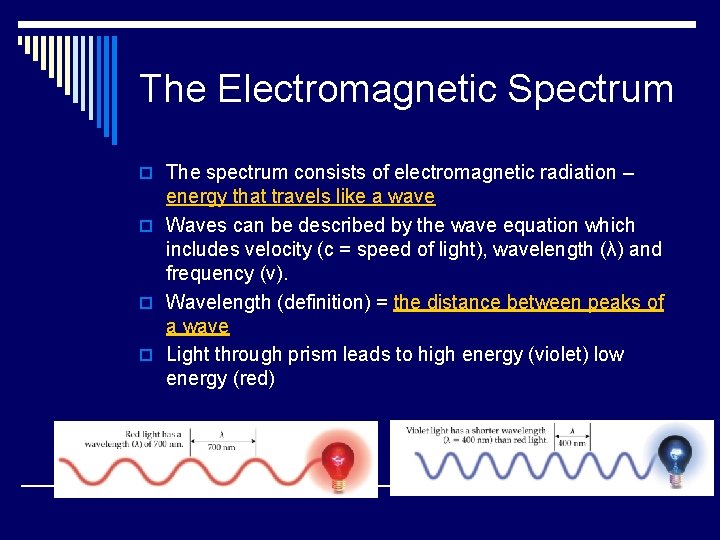
The Electromagnetic Spectrum o The spectrum consists of electromagnetic radiation – energy that travels like a wave o Waves can be described by the wave equation which includes velocity (c = speed of light), wavelength (λ) and frequency (ν). o Wavelength (definition) = the distance between peaks of a wave o Light through prism leads to high energy (violet) low energy (red)

The Electromagnetic Spectrum o ROYGBIV - colors of the visible spectrum o Bright Line Spectrum (BLS) – caused by e- emitting o o o energy as they return to lower energy levels energy level. heat sodium - yellow light 2 c heat lithium - red light elements can appear to give off the same color light, but each will have its own BLS - used to determine identity of an element BLS - validates Bohr’s idea that electrons jump to different energy levels and give off different wavelengths of light

The Electromagnetic Spectrum o Light from the sun (white light) appears as a continuous spectrum of light. o Continuous Spectrum of Light (definition) = There are no discrete, individual wavelengths of light but rather all wavelengths appear, one after the other in a continuous fashion o Spectroscopy (definition) = the study of substances from the light they emit. o We will use spectroscopes (An instrument that splits light into its component colors) and flame tests to study elements because each element emits a different spectrum of light when exited.

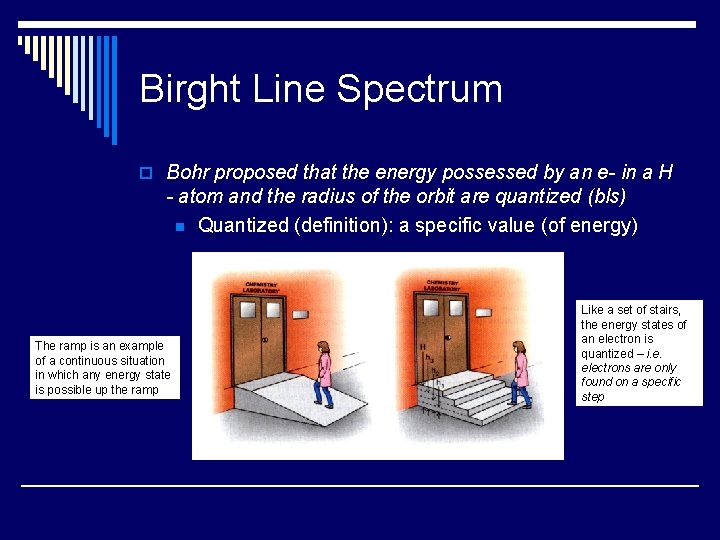
Birght Line Spectrum o Bohr proposed that the energy possessed by an e- in a H - atom and the radius of the orbit are quantized (bls) n Quantized (definition): a specific value (of energy) The ramp is an example of a continuous situation in which any energy state is possible up the ramp Like a set of stairs, the energy states of an electron is quantized – i. e. electrons are only found on a specific step

Bohr’s Energy Absorption Process o Light or energy excites an e- from a lower energy level (e- shell) to a higher energy level o These energy levels are “ quantized “ (the e- cannot be in between levels), the e- disappears from one shell and reappears in another o This absorption or excitation process is called a quantum leap or quantum jump

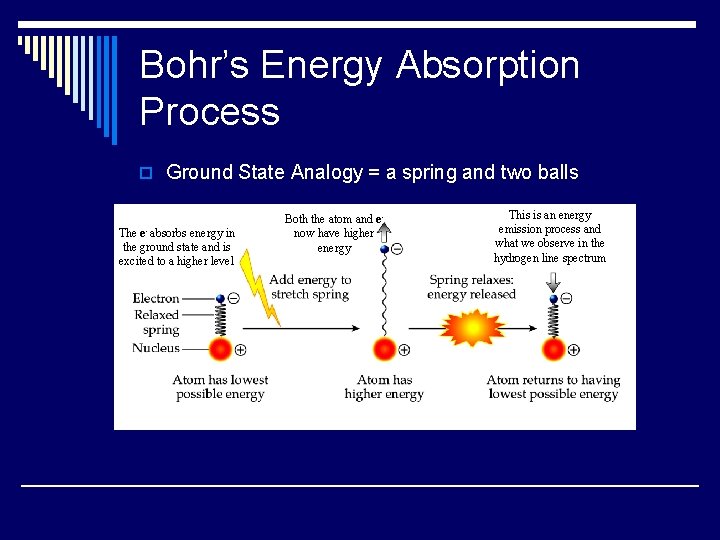
Bohr’s Energy Absorption Process o Ground State Analogy = a spring and two balls The e- absorbs energy in the ground state and is excited to a higher level Both the atom and enow have higher energy This is an energy emission process and what we observe in the hydrogen line spectrum

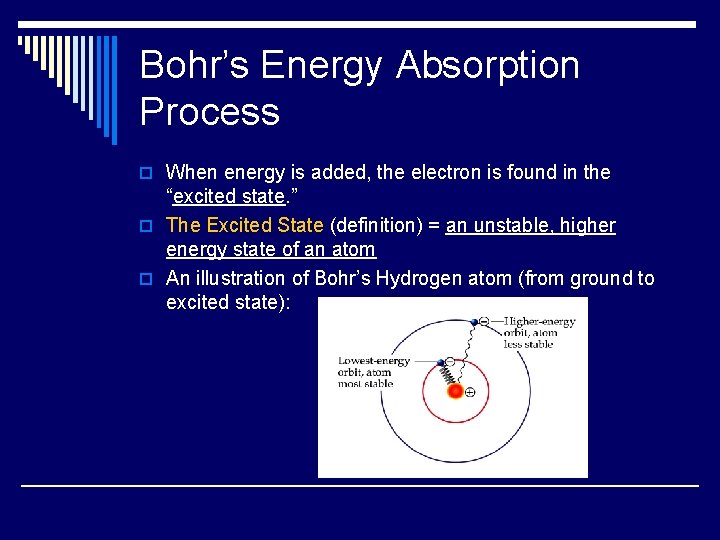
Bohr’s Energy Absorption Process o When energy is added, the electron is found in the “excited state. ” o The Excited State (definition) = an unstable, higher energy state of an atom o An illustration of Bohr’s Hydrogen atom (from ground to excited state):

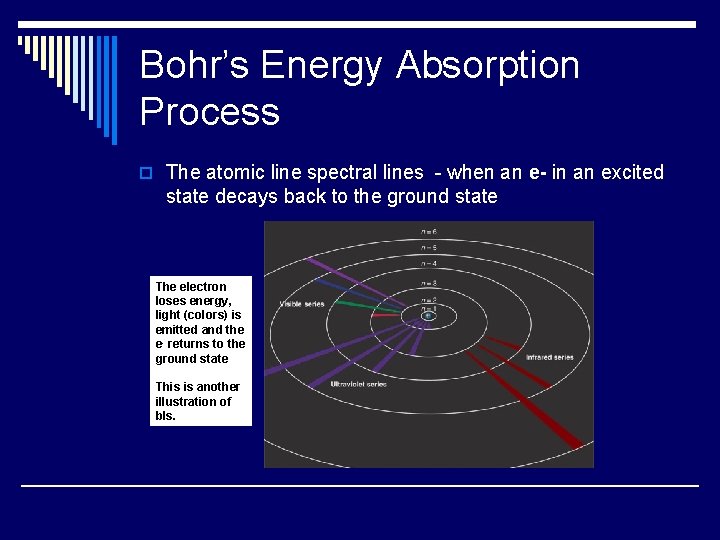
Bohr’s Energy Absorption Process o The atomic line spectral lines - when an e- in an excited state decays back to the ground state The electron loses energy, light (colors) is emitted and the e- returns to the ground state This is another illustration of bls.

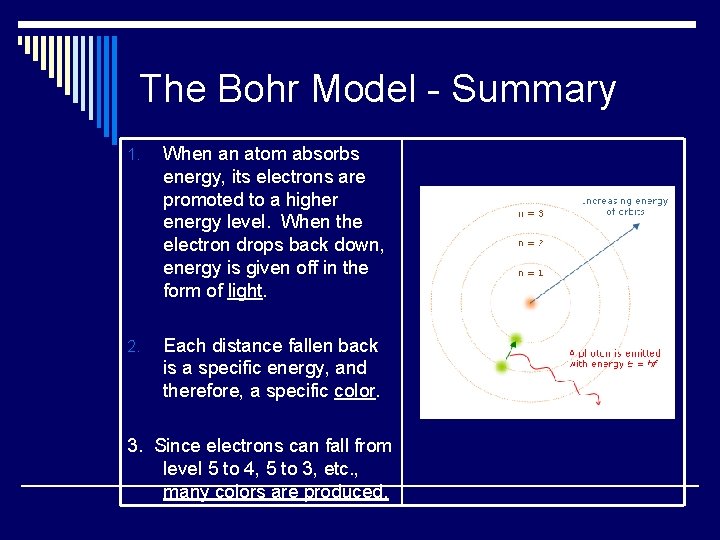
The Bohr Model - Summary 1. When an atom absorbs energy, its electrons are promoted to a higher energy level. When the electron drops back down, energy is given off in the form of light. 2. Each distance fallen back is a specific energy, and therefore, a specific color. 3. Since electrons can fall from level 5 to 4, 5 to 3, etc. , many colors are produced.

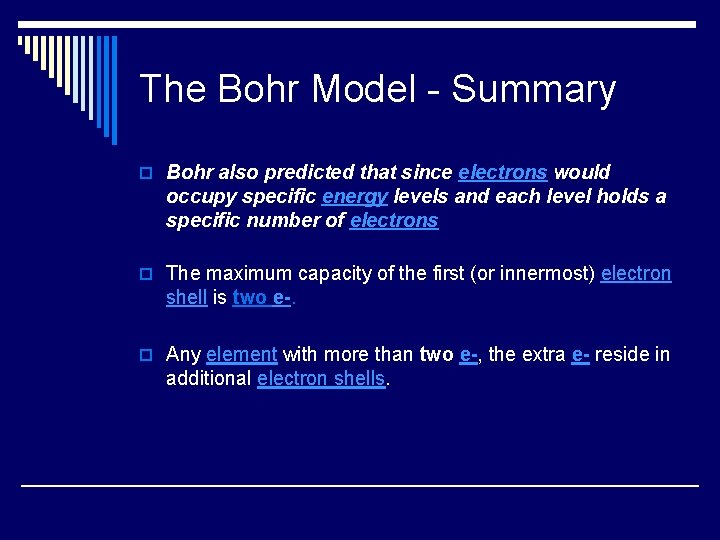
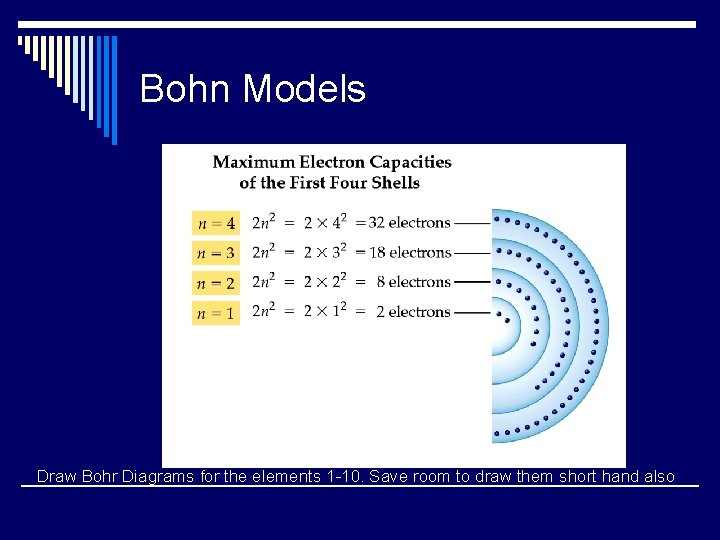
The Bohr Model - Summary o Bohr also predicted that since electrons would occupy specific energy levels and each level holds a specific number of electrons o The maximum capacity of the first (or innermost) electron shell is two e-. o Any element with more than two e-, the extra e- reside in additional electron shells.

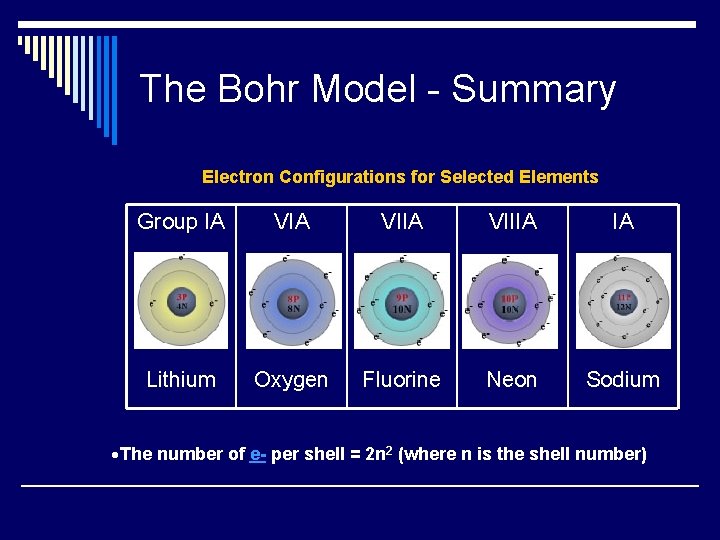
The Bohr Model - Summary Electron Configurations for Selected Elements Group IA VIIA VIIIA IA Lithium Oxygen Fluorine Neon Sodium The number of e- per shell = 2 n 2 (where n is the shell number)

Bohn Models Draw Bohr Diagrams for the elements 1 -10. Save room to draw them short hand also

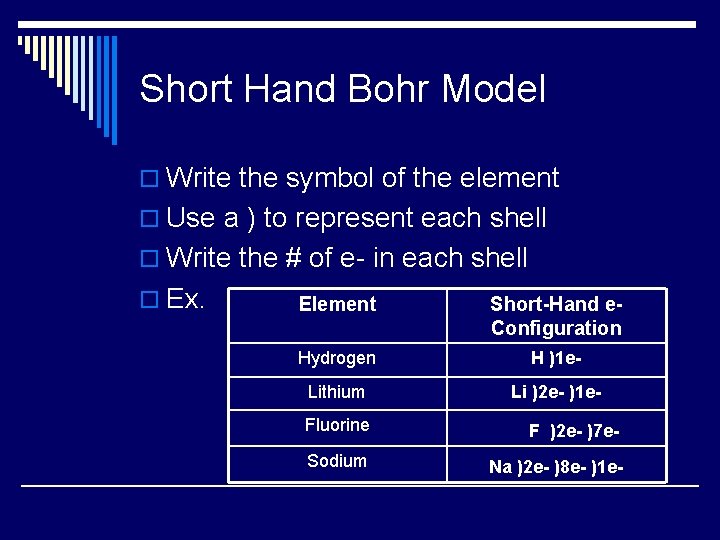
Short Hand Bohr Model o Write the symbol of the element o Use a ) to represent each shell o Write the # of e- in each shell o Ex. Element Short-Hand e- Configuration Hydrogen H )1 e- Lithium Li )2 e- )1 e- Fluorine F )2 e- )7 e- Sodium Na )2 e- )8 e- )1 e-

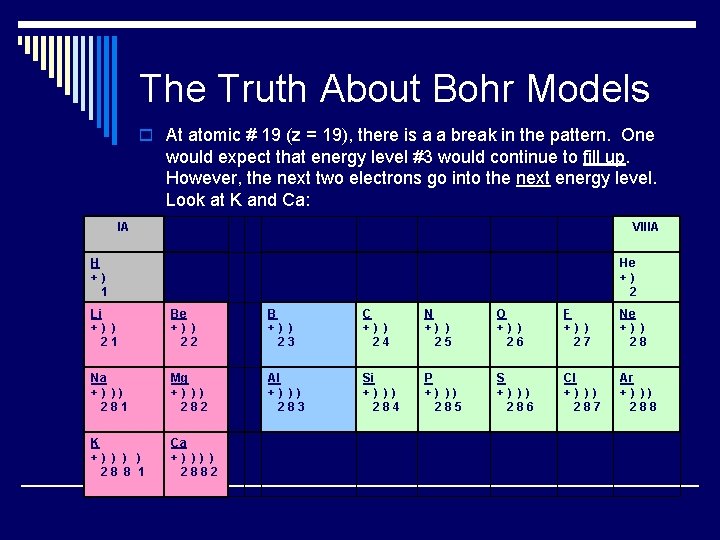
The Truth About Bohr Models o At atomic # 19 (z = 19), there is a a break in the pattern. One would expect that energy level #3 would continue to fill up. However, the next two electrons go into the next energy level. Look at K and Ca: IA H + ) 1 VIIIA IVA VA VIIA He + ) 2 Li + ) ) 2 1 Be + ) ) 2 2 B + ) ) 2 3 C + ) ) 2 4 N + ) ) 2 5 O + ) ) 2 6 F + ) ) 2 7 Ne + ) ) 2 8 Na + ) ) ) 2 8 1 Mg + ) ) ) 2 8 2 Al + ) ) ) 2 8 3 Si + ) ) ) 2 8 4 P + ) ) ) 2 8 5 S + ) ) ) 2 8 6 Cl + ) ) ) 2 8 7 Ar + ) ) ) 2 8 8 K + ) ) ) ) 2 8 8 1 Ca + ) ) 2 8 8 2

The Truth Continued…… o So, there is a relationship between the main column o o # and the number of outershell electrons. Column # = the number of valence electrons And, there is a relationship between the row # and the number of energy levels. Row # = the number of shells The Bohr model truly works well for the H atom only n for elements larger than H the model does not work.

Bohr Summed Up o Bohr made 2 huge contributions to the development of modern atom theory n n He explained the atomic line spectra in terms of electron energies He introduced the idea of quantized electron energy levels in the atom o The Bohr atom lasted for about 13 years and was quickly replaced by the quantum mechanical model of the atom. The Bohr model is a good starting point for understanding the quantum mechanical model of the atom Do ws# 1, question 1 - Use short-hand configuration

o 4. 2 Quantum Numbers and Atomic Orbitals o 4. 3 Electron Configuration

Quantum Numbers & Atomic Orbitals o The Bohr model describes the atom as having definite orbitals occupied by electron particles. o Schrödinger (1926) introduced wave mechanics to describe electrons – proved Bohr’s Model to be a lie n Based his idea that electrons behaved like light (photons). n Electrons show diffraction (interference) properties like light. n Treats electrons as waves that are found in orbitals. n Orbitals (definition) = clouds that show region of probable location of a particular electron.

Wave Mechanical Model o The Bohr model really is the wave mechanical model o There are many types of orbitals – we can see them on the periodic table


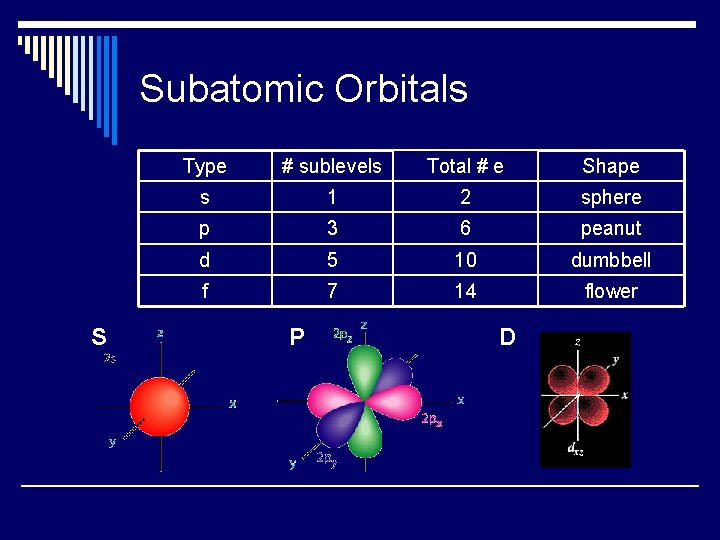
Subatomic Orbitals S Type # sublevels Total # e Shape s 1 2 sphere p 3 6 peanut d 5 10 dumbbell f 7 14 flower P D

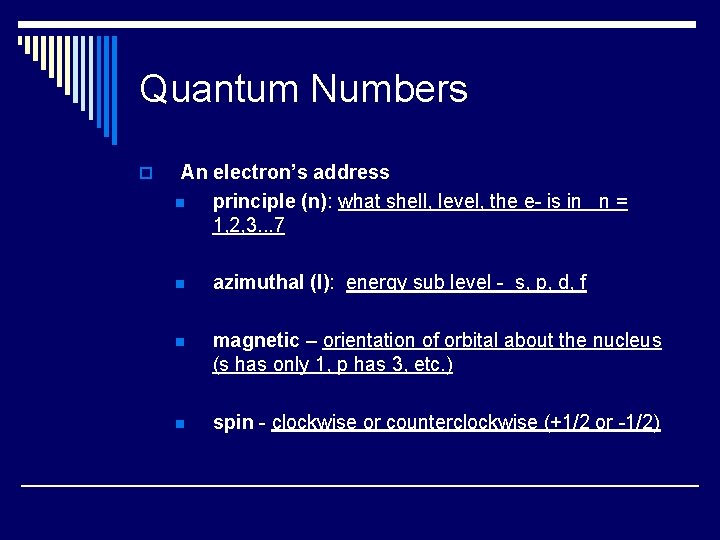
Quantum Numbers o An electron’s address n principle (n): what shell, level, the e- is in n = 1, 2, 3. . . 7 n azimuthal (l): energy sub level - s, p, d, f n magnetic – orientation of orbital about the nucleus (s has only 1, p has 3, etc. ) n spin - clockwise or counterclockwise (+1/2 or -1/2)

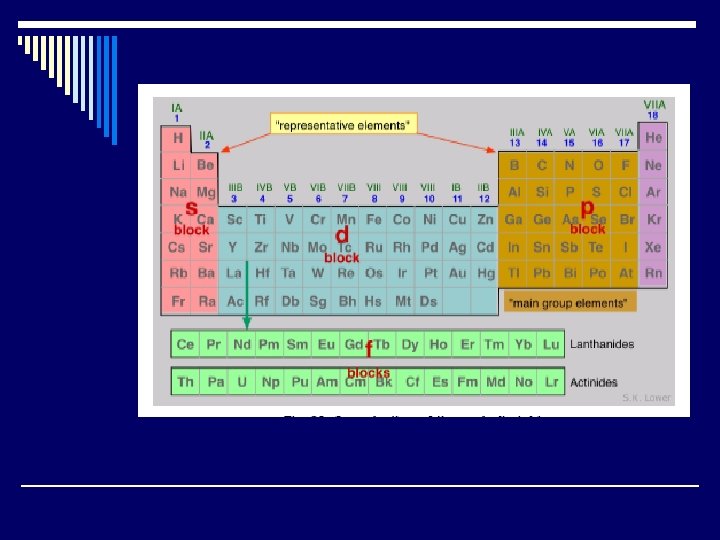
Label Your Periodic Tabel o On your periodic table, shade azimuthal s, p, d, f blocks different colors o Label the principal quantum numbers… 1 - 7 o Label the valence electrons across the top

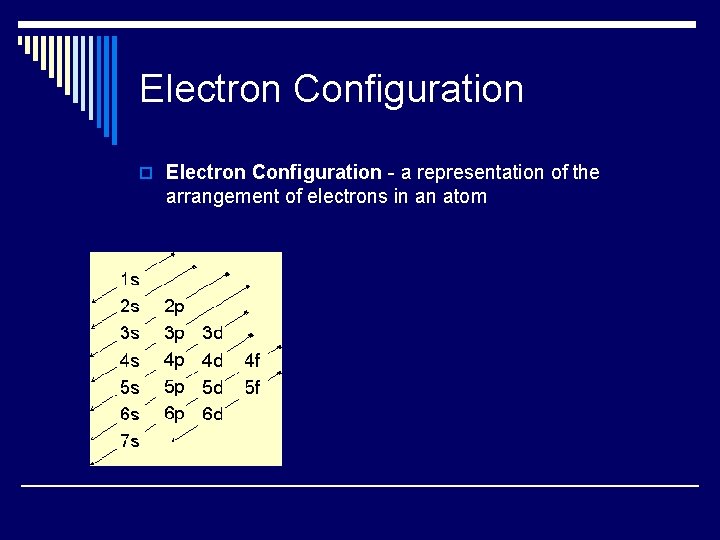
Electron Configuration o Electron Configuration - a representation of the arrangement of electrons in an atom

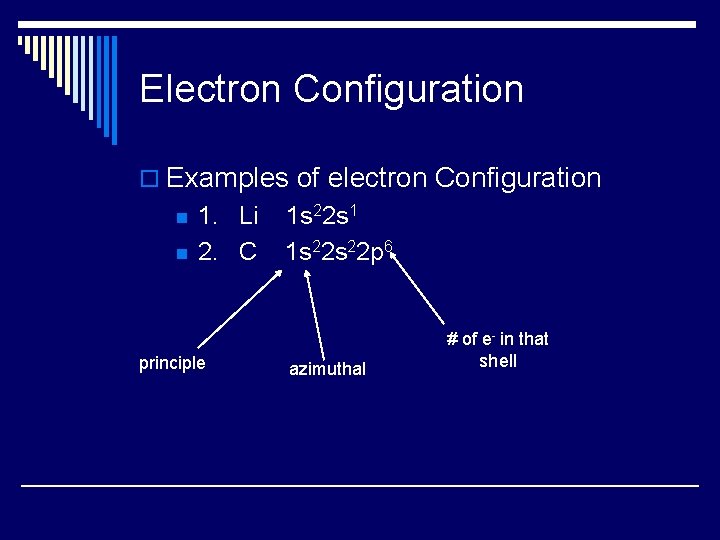
Electron Configuration o Examples of electron Configuration n n 1. Li 2. C principle 1 s 22 s 1 1 s 22 p 6 azimuthal # of e- in that shell

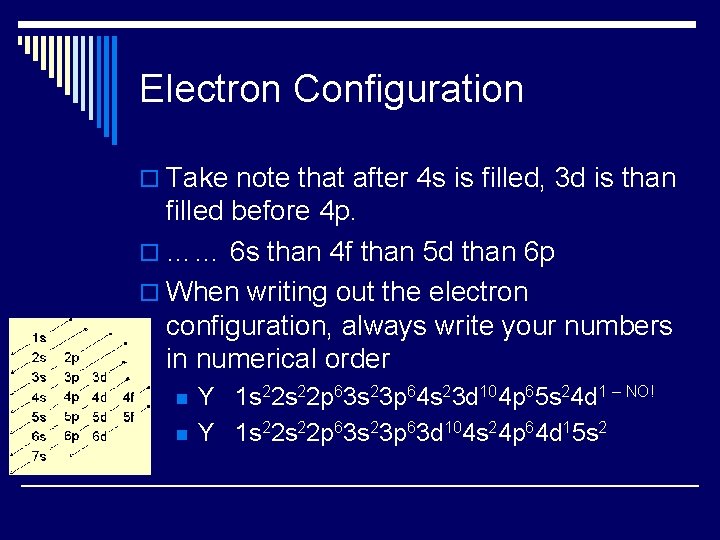
Electron Configuration o Take note that after 4 s is filled, 3 d is than filled before 4 p. o …… 6 s than 4 f than 5 d than 6 p o When writing out the electron configuration, always write your numbers in numerical order n n Y 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 24 d 1 – NO! Y 1 s 22 p 63 s 23 p 63 d 104 s 24 p 64 d 15 s 2

Electron Configuration o Examples: o Be o. O o Ca o Mn

Electron Configuration o Examples o Pb o Os

Electron Configuration o Short Hand n n Write the name of the last noble gas Write the electron config. that follows p Ex. n Fe [Ar]3 d 64 s 2 Exceptions p Cr [Ar] 3 d 54 s 1 p Cu, Ag, Au- all s’s donate 1 e- to make the d orbital full n Cu [Ar] 4 s 13 d 10

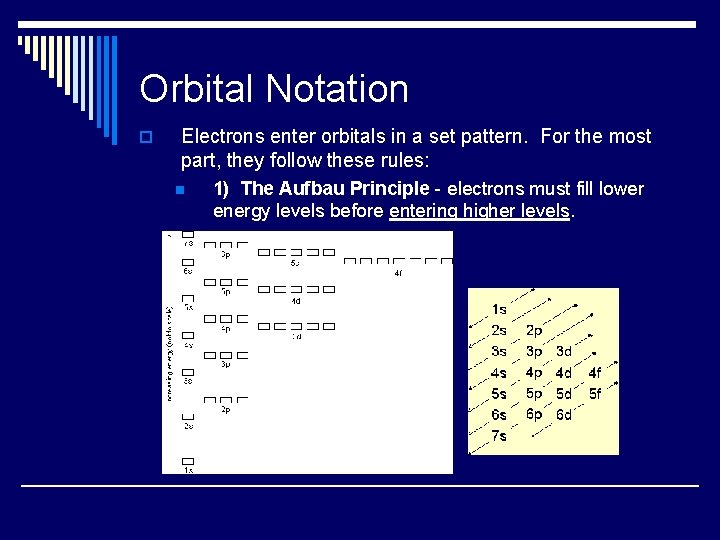
Orbital Notation o Electrons enter orbitals in a set pattern. For the most part, they follow these rules: n 1) The Aufbau Principle - electrons must fill lower energy levels before entering higher levels.

Orbital Notation o Orbitals are like "rooms" within which electrons "reside". o The s subshell has one s-orbital. The p subshell has three p-orbitals. The d subshell has 5 and f has 7. o Each orbital can hold at most 2 electrons

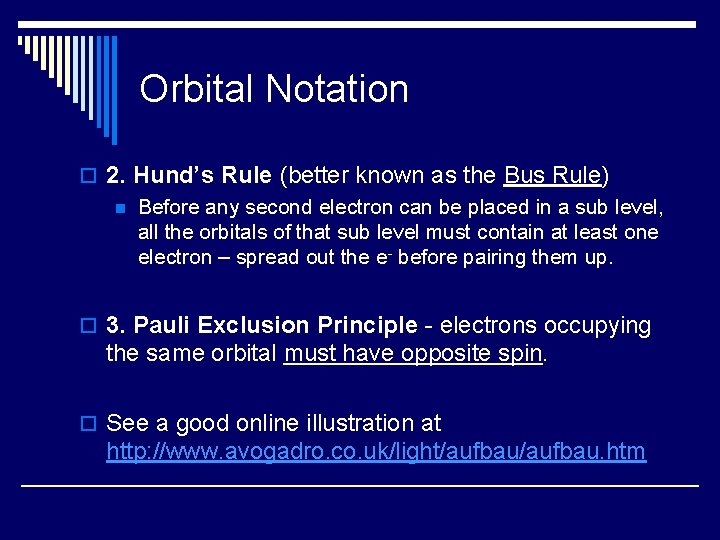
Orbital Notation o 2. Hund’s Rule (better known as the Bus Rule) n Before any second electron can be placed in a sub level, all the orbitals of that sub level must contain at least one electron – spread out the e- before pairing them up. o 3. Pauli Exclusion Principle - electrons occupying the same orbital must have opposite spin. o See a good online illustration at http: //www. avogadro. co. uk/light/aufbau. htm

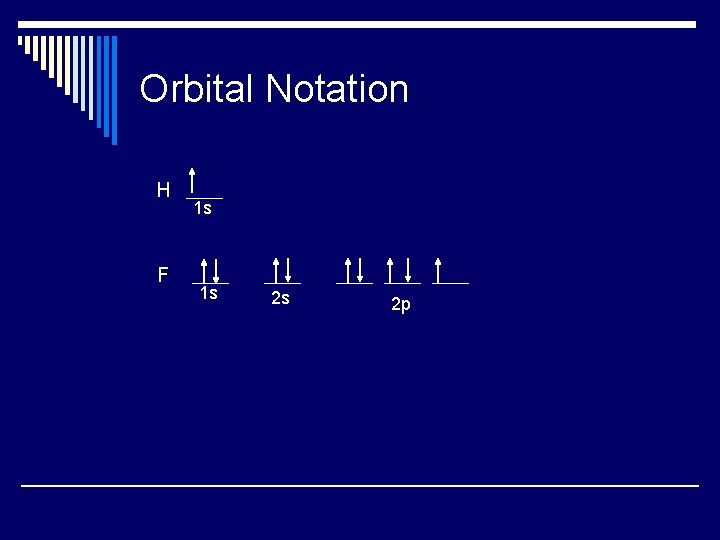
Orbital Notation H F 1 s 1 s 2 s 2 p

Orbital Notation o Examples: o Li F o Na Sc

Orbital Notation o We can also do shorthand orbital notation (outer shell only) Ag [Kr] 4 d 105 s 1 Ag [Kr] 4 d o Ca o Fe 5 s N

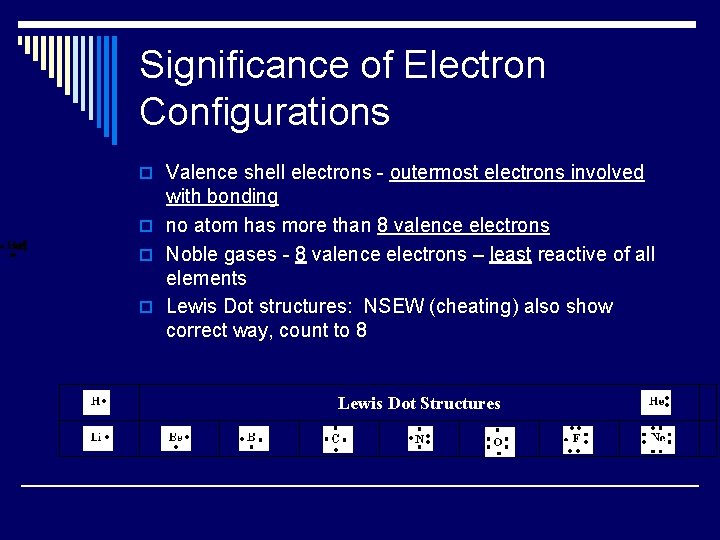
Significance of Electron Configurations o Valence shell electrons - outermost electrons involved with bonding o no atom has more than 8 valence electrons o Noble gases - 8 valence electrons – least reactive of all elements o Lewis Dot structures: NSEW (cheating) also show correct way, count to 8 Lewis Dot Structures