Chapter 4 Aqueous Reactions AP Chemistry Unit 2








- Slides: 19

Chapter 4: Aqueous Reactions AP Chemistry Unit 2

Water • One of the most important substances on Earth. • Can dissolve many different substances. • Known as the “universal solvent” • A polar molecule because of its unequal charge distribution. 2

Dissolving Process • dissolution solid liquid

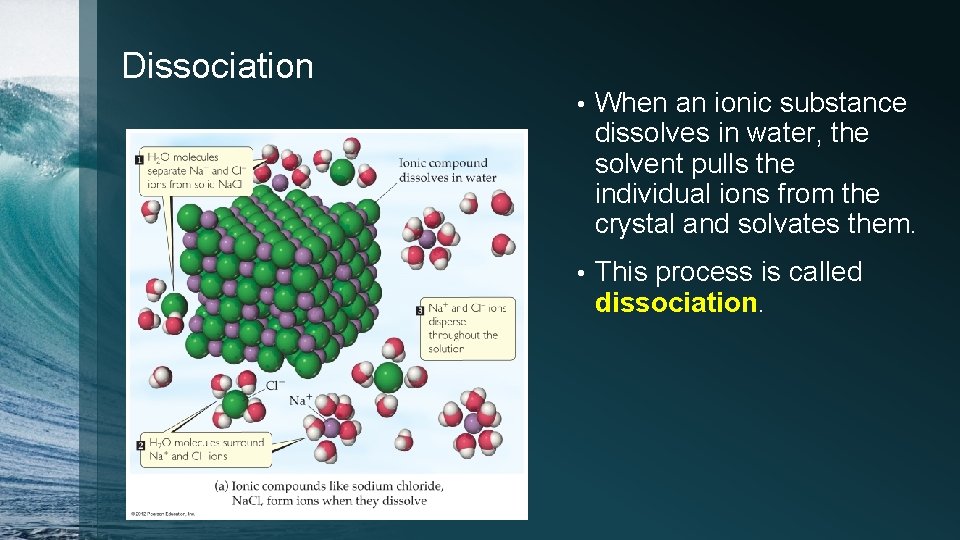
Dissociation • When an ionic substance dissolves in water, the solvent pulls the individual ions from the crystal and solvates them. • This process is called dissociation.

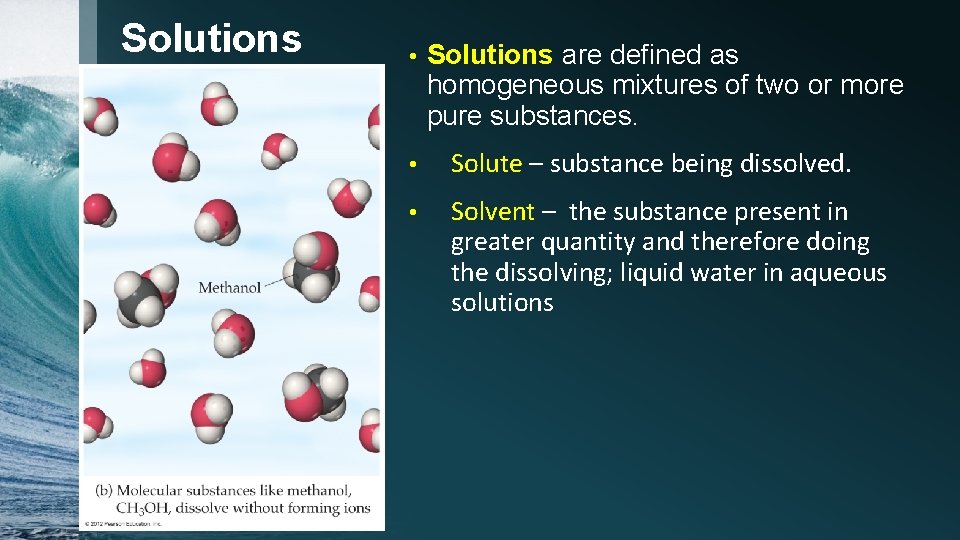
Solutions © 2012 Pearson Education, Inc. • Solutions are defined as homogeneous mixtures of two or more pure substances. • Solute – substance being dissolved. • Solvent – the substance present in greater quantity and therefore doing the dissolving; liquid water in aqueous solutions

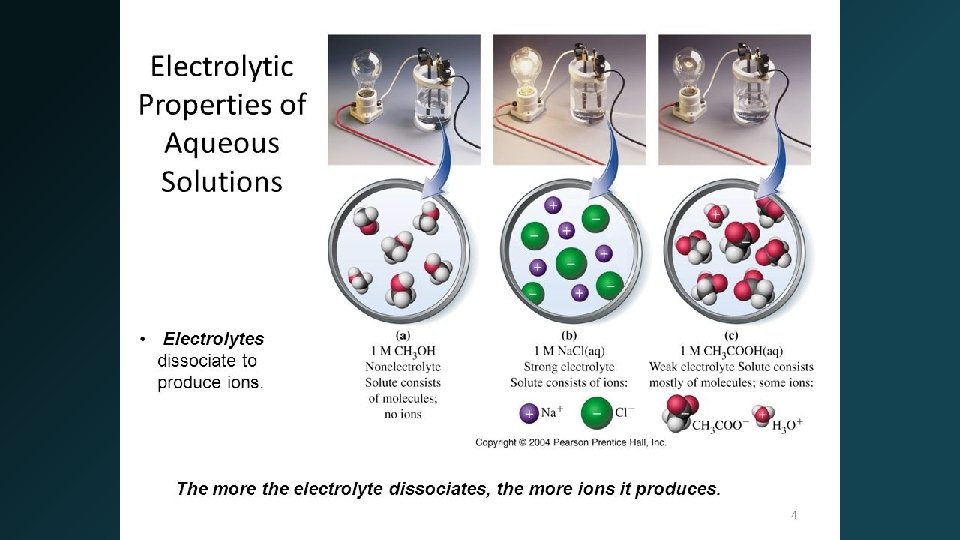
Electrolytes • Electrolyte – substance that when dissolved in water produces a solution that can conduct electricity. • Strong Electrolytes – conduct current very efficiently (bulb shines brightly). Completely ionized in water. • Weak Electrolytes – conduct only a small current (bulb glows dimly). A small degree of ionization in water. • Nonelectrolytes – no current flows (bulb remains unlit). Dissolves but does not produce any ions. 6

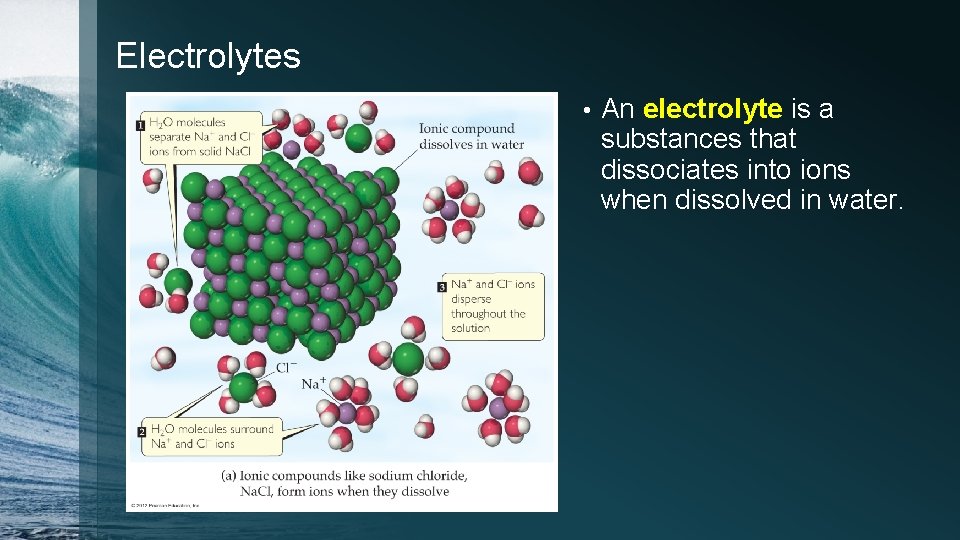
Electrolytes • An electrolyte is a substances that dissociates into ions when dissolved in water.


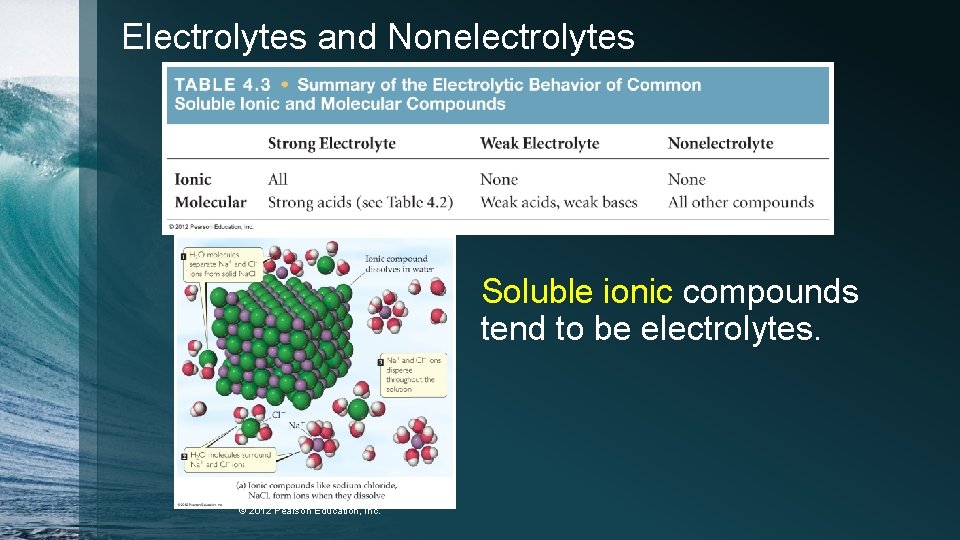
Electrolytes and Nonelectrolytes Soluble ionic compounds tend to be electrolytes. © 2012 Pearson Education, Inc.

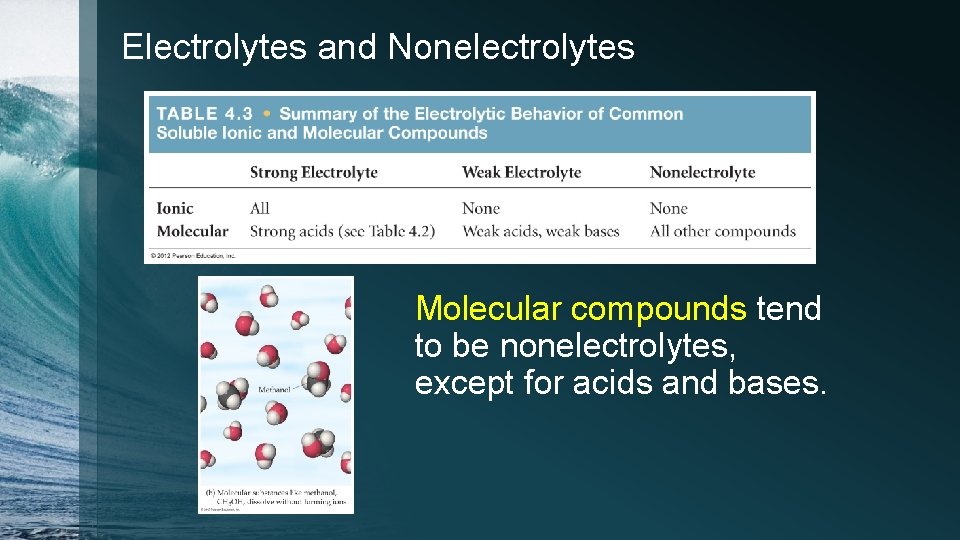
Electrolytes and Nonelectrolytes Molecular compounds tend to be nonelectrolytes, except for acids and bases.

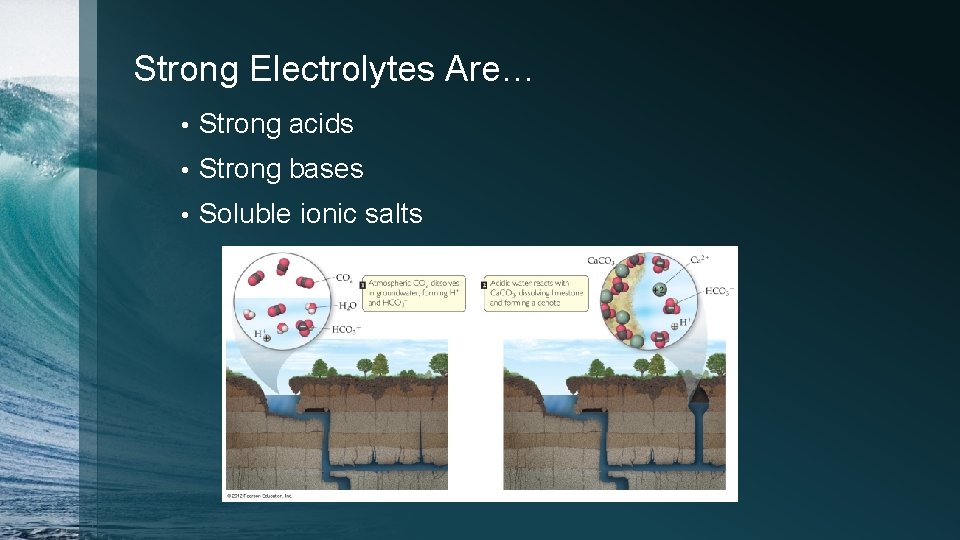
Strong Electrolytes Are… • Strong acids • Strong bases • Soluble ionic salts

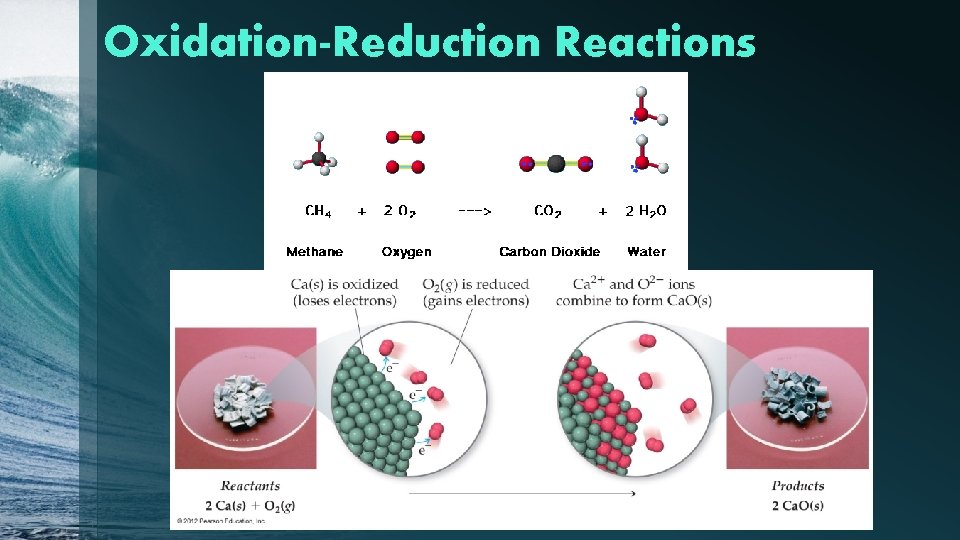
Oxidation-Reduction Reactions

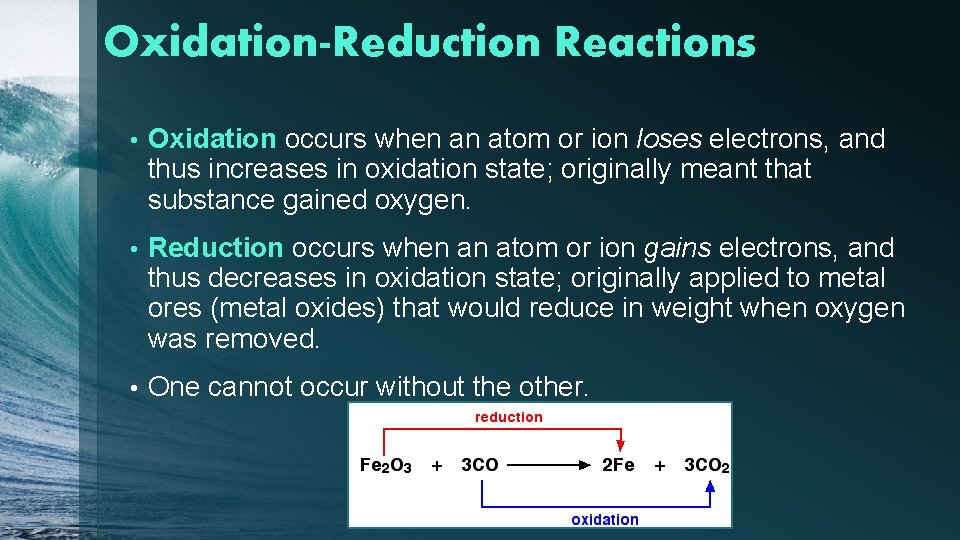
Oxidation-Reduction Reactions • Oxidation occurs when an atom or ion loses electrons, and thus increases in oxidation state; originally meant that substance gained oxygen. • Reduction occurs when an atom or ion gains electrons, and thus decreases in oxidation state; originally applied to metal ores (metal oxides) that would reduce in weight when oxygen was removed. • One cannot occur without the other.

Oxidation Numbers • arbitrary positive/negative numbers which indicate the extent to which redox has occurred to specific atoms • Assigned to elements COVALENT molecules and also ions • • Assigns hypothetical charges to all atoms as if all bonds are ionic (electrons shifted completely) Based on EN of atom (its tendency to gain electrons in a chemical bond)

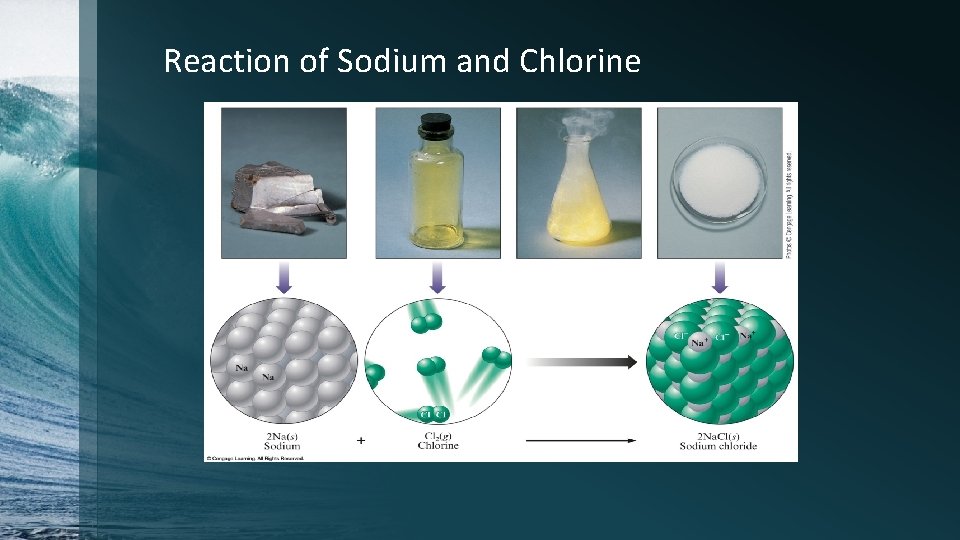
Reaction of Sodium and Chlorine

Rules for Assigning Oxidation States 1. Oxidation state of an atom in elemental form = 0 2. Oxygen = -2 in covalent compounds (except in peroxides where it = -1, and when paired with F) 3. Hydrogen = +1 in most compounds (except in hydrides where it = 1) 4. Fluorine = -1 in all compounds (most EN element) 5. Sum of oxidation states = 0 in compounds 6. Sum of oxidation states = charge of the ion in ions 7. Oxidation state of alkali metals in compound is +1

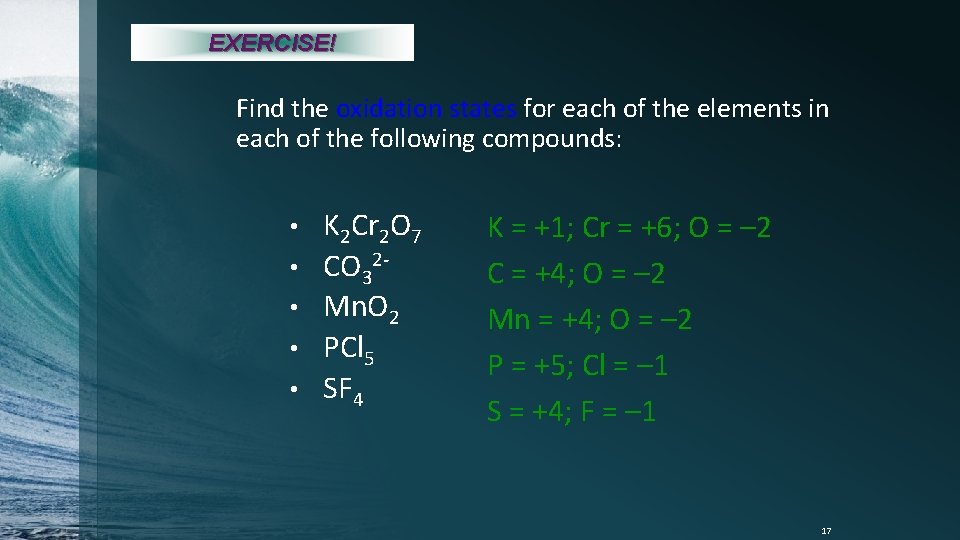
EXERCISE! Find the oxidation states for each of the elements in each of the following compounds: • • • K 2 Cr 2 O 7 CO 32 Mn. O 2 PCl 5 SF 4 K = +1; Cr = +6; O = – 2 C = +4; O = – 2 Mn = +4; O = – 2 P = +5; Cl = – 1 S = +4; F = – 1 17

Oxidizing Agent vs. Reducing Agent • Oxidizing Agent – the reactant that causes oxidation in another substance (and thereby contains the element that is reduced) • Reducing Agent – the reactant that causes reduction in another substance (and thereby contains the element that is oxidized)

CONCEPT CHECK! Which of the following are oxidation-reduction reactions? Identify the oxidizing agent and the reducing agent. a)Zn(s) + 2 HCl(aq) Zn. Cl 2(aq) + H 2(g) b)Cr 2 O 72 -(aq) + 2 OH-(aq) c)2 Cu. Cl(aq) 2 Cr. O 42 -(aq) + H 2 O(l) Cu. Cl 2(aq) + Cu(s)