Chapter 4 4 2 Types of Chemical Reactions


- Slides: 10

Chapter 4 (4. 2) Types of Chemical Reactions - Part 2

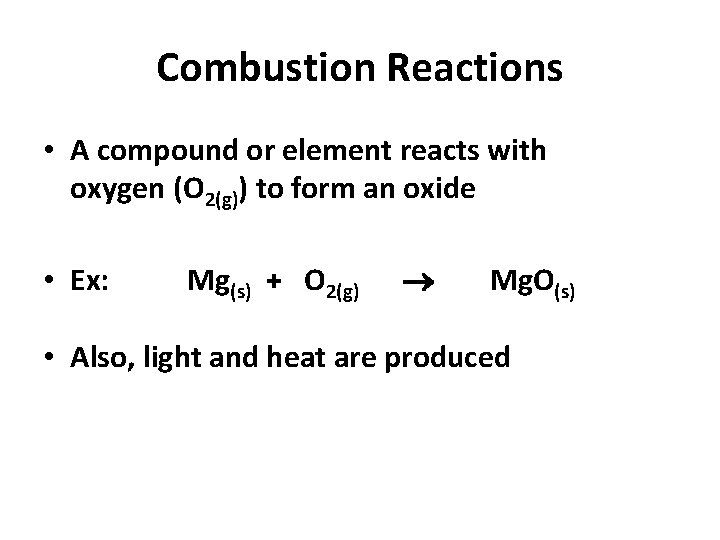
Combustion Reactions • A compound or element reacts with oxygen (O 2(g)) to form an oxide • Ex: Mg(s) + O 2(g) Mg. O(s) • Also, light and heat are produced

• A hydrocarbon is a compound that is composed only of the elements carbon and hydrogen • Ex: methane gas CH 4(g) • Combustion of a hydrocarbon produces the compounds carbon dioxide and water. • Ex: CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(g)

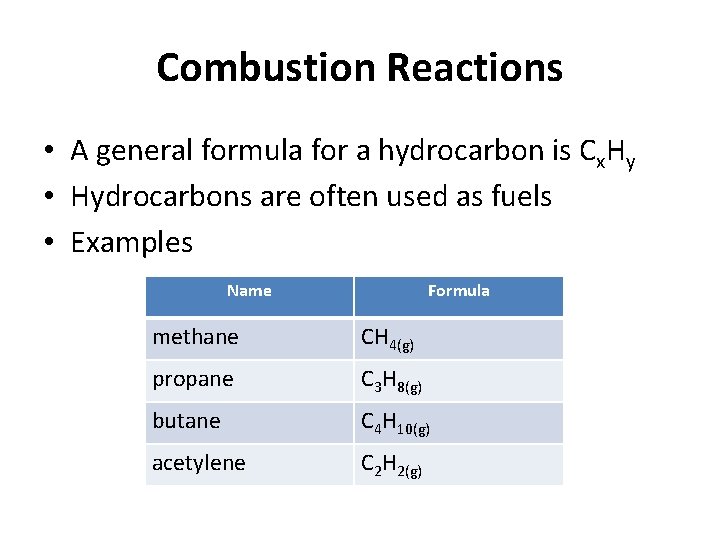
Combustion Reactions • A general formula for a hydrocarbon is Cx. Hy • Hydrocarbons are often used as fuels • Examples Name Formula methane CH 4(g) propane C 3 H 8(g) butane C 4 H 10(g) acetylene C 2 H 2(g)

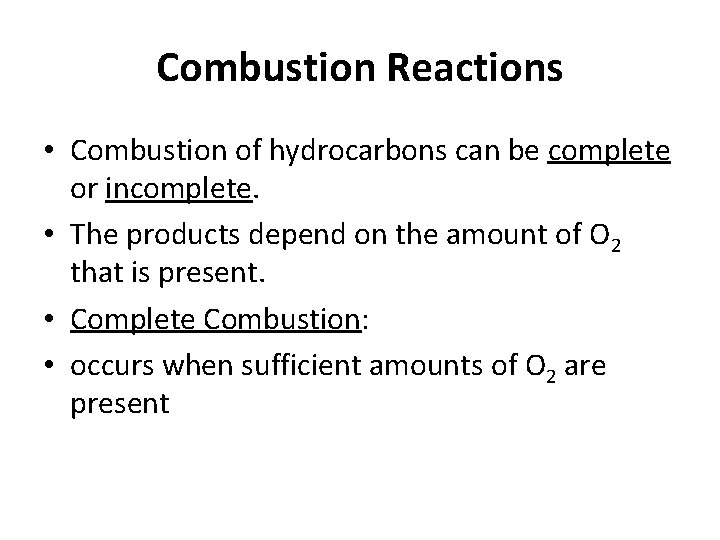
Combustion Reactions • Combustion of hydrocarbons can be complete or incomplete. • The products depend on the amount of O 2 that is present. • Complete Combustion: • occurs when sufficient amounts of O 2 are present

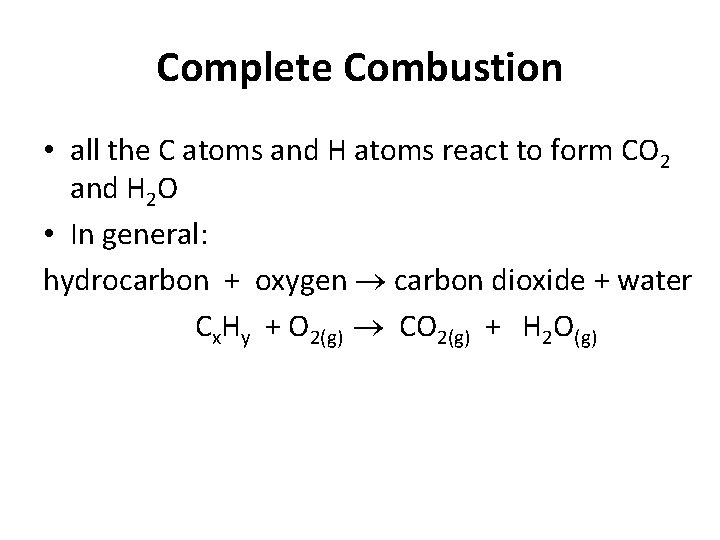
Complete Combustion • all the C atoms and H atoms react to form CO 2 and H 2 O • In general: hydrocarbon + oxygen carbon dioxide + water Cx. Hy + O 2(g) CO 2(g) + H 2 O(g)

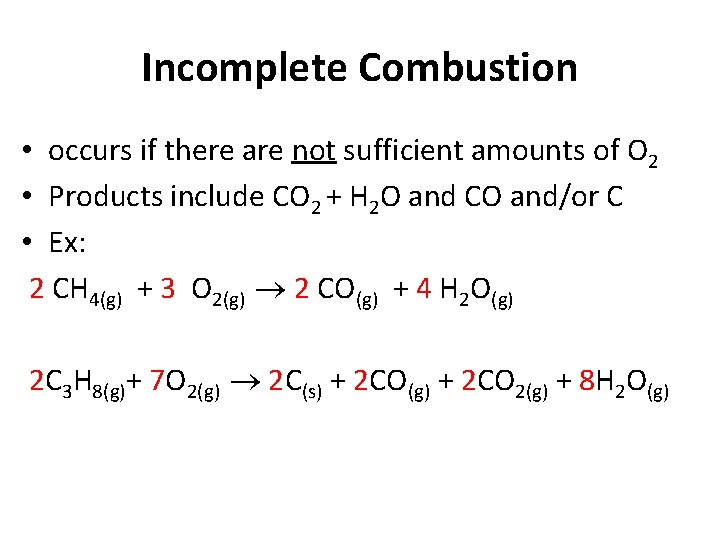
Incomplete Combustion • occurs if there are not sufficient amounts of O 2 • Products include CO 2 + H 2 O and CO and/or C • Ex: 2 CH 4(g) + 3 O 2(g) 2 CO(g) + 4 H 2 O(g) 2 C 3 H 8(g)+ 7 O 2(g) 2 C(s) + 2 CO(g) + 2 CO 2(g) + 8 H 2 O(g)

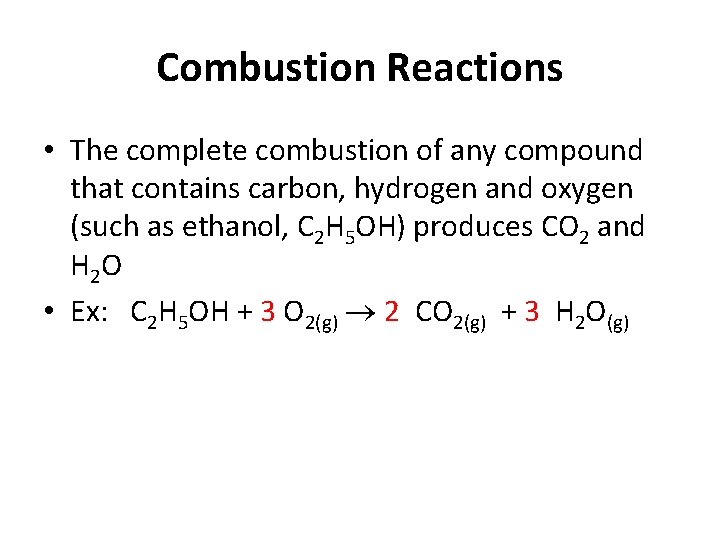
Combustion Reactions • The complete combustion of any compound that contains carbon, hydrogen and oxygen (such as ethanol, C 2 H 5 OH) produces CO 2 and H 2 O • Ex: C 2 H 5 OH + 3 O 2(g) 2 CO 2(g) + 3 H 2 O(g)

Testing for Gases • The presence of gases may be detected using chemical reactions: • Carbon Dioxide Gas - turns clear limewater cloudy - burning wooden splint goes out • Hydrogen Gas - burning wooden splint burns with a “pop” sound • Oxygen Gas - glowing wooden splint relights

Homework • Text p 124 #17 -20 P 125 #4 p 584 #1 a, 2, 4 p 587 # 1, 3, 4 Reminder: Inv 4 – lab report (not formal) due tomorrow