Chapter 4 1 Atoms and the Periodic Table

Chapter 4 – 1 Atoms and the Periodic Table Atomic Structure

What are atoms? n n n Democritus – universe made of invisible units called atoms Atom – “unable to divide” No real proof

Atomic Theory n John Dalton – atoms cannot be divided n n n 1800’s – English School Teacher Atoms of a given element are alike Atoms of different elements can form compounds
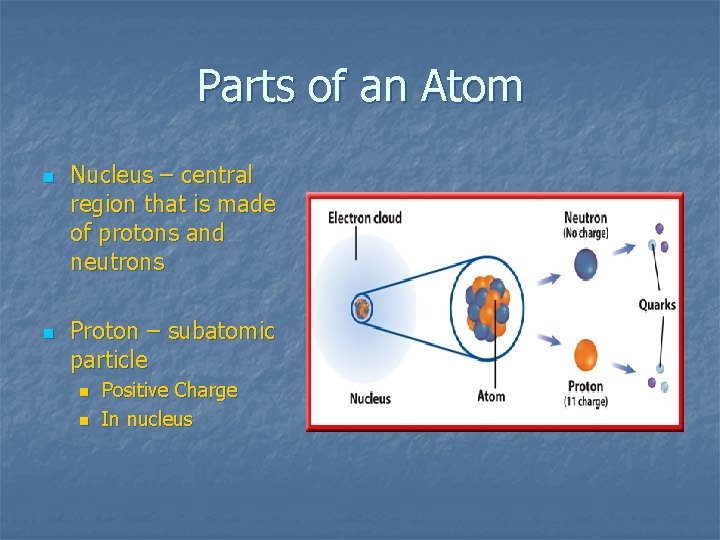
Parts of an Atom n n Nucleus – central region that is made of protons and neutrons Proton – subatomic particle n n Positive Charge In nucleus
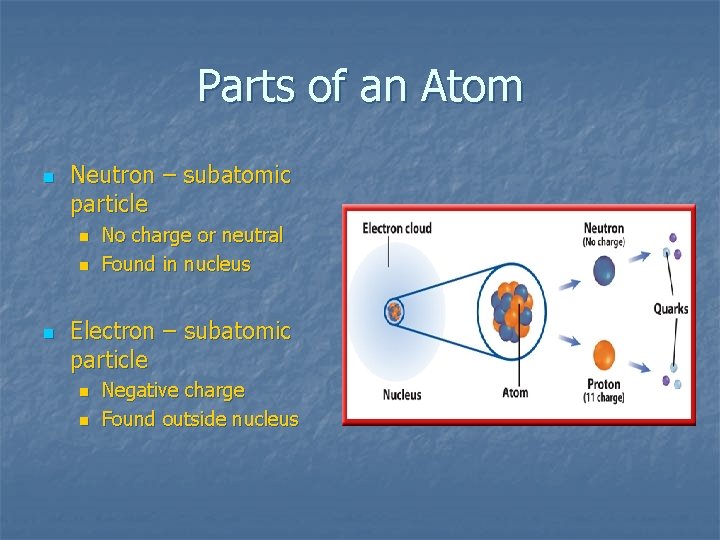
Parts of an Atom n Neutron – subatomic particle n n n No charge or neutral Found in nucleus Electron – subatomic particle n n Negative charge Found outside nucleus

Quarks n Protons and Neutrons are made of smaller particles called quarks.
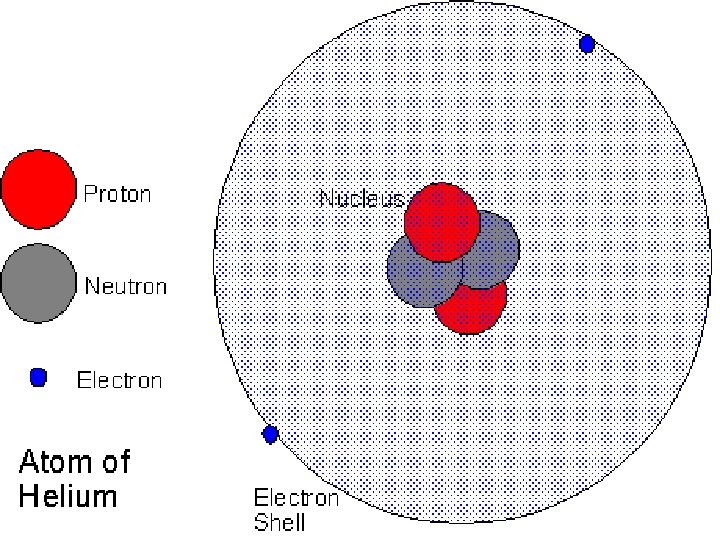
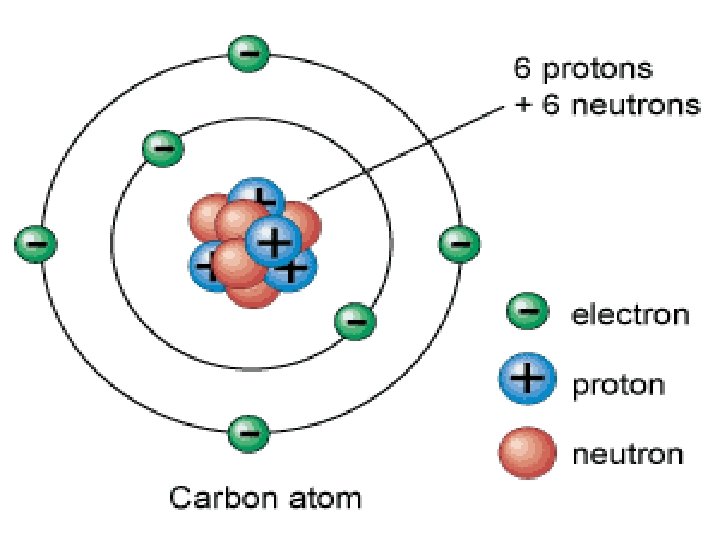
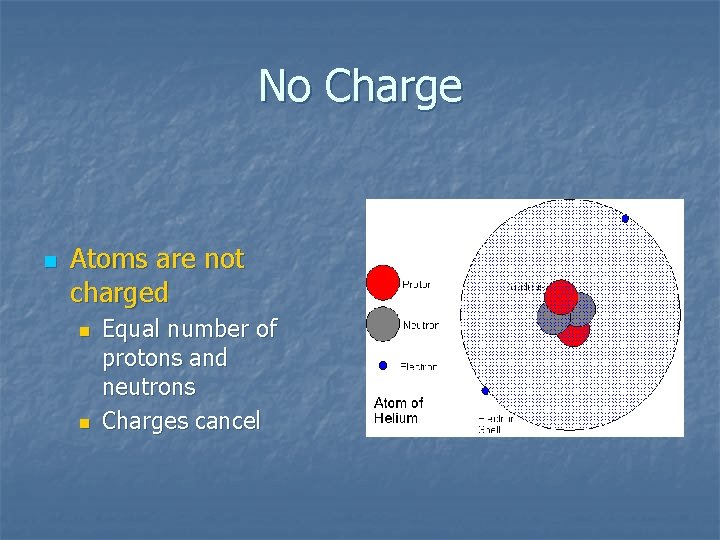
No Charge n Atoms are not charged n n Equal number of protons and neutrons Charges cancel
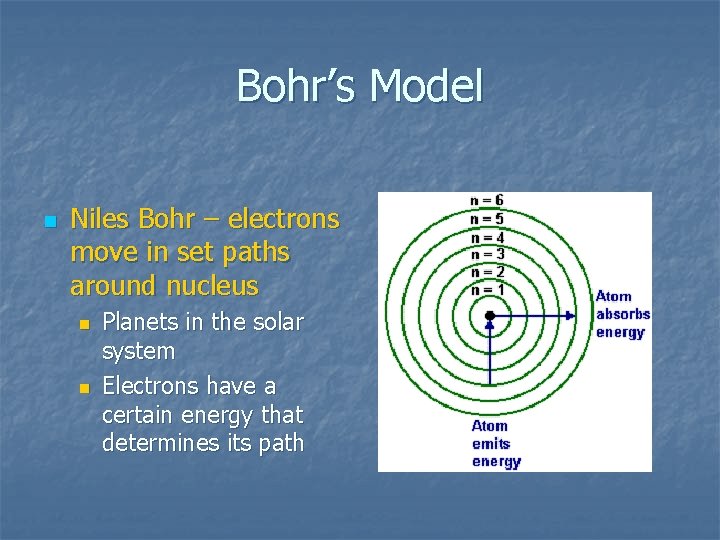
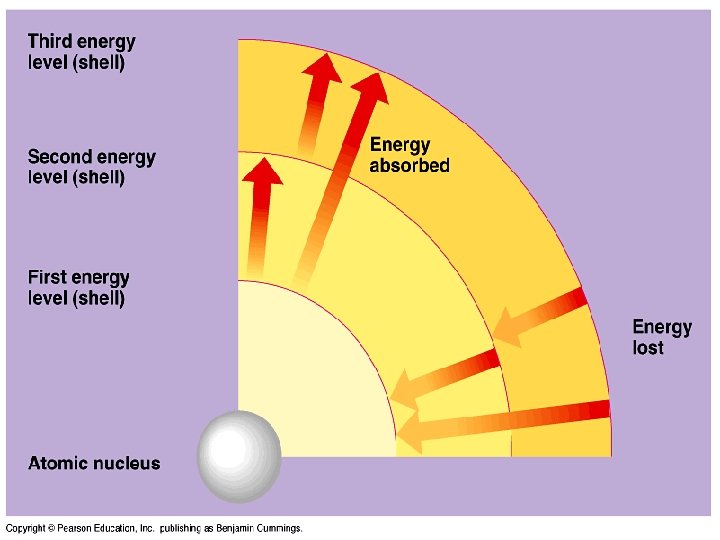
Bohr’s Model n Niles Bohr – electrons move in set paths around nucleus n n Planets in the solar system Electrons have a certain energy that determines its path
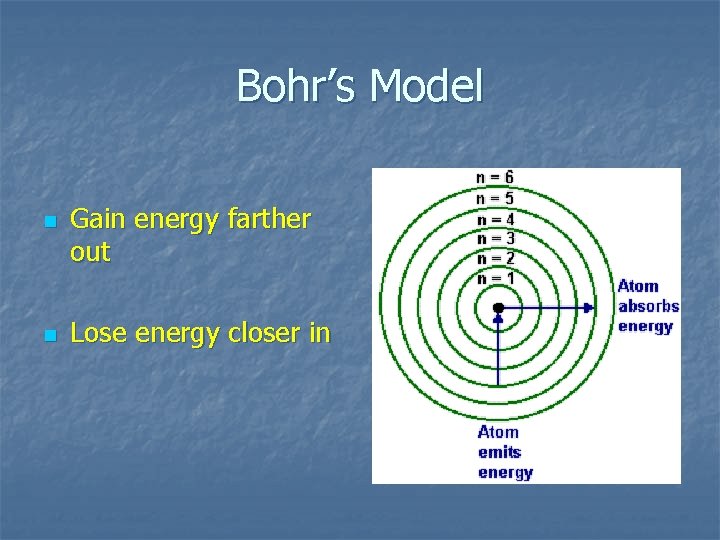
Bohr’s Model n n Gain energy farther out Lose energy closer in
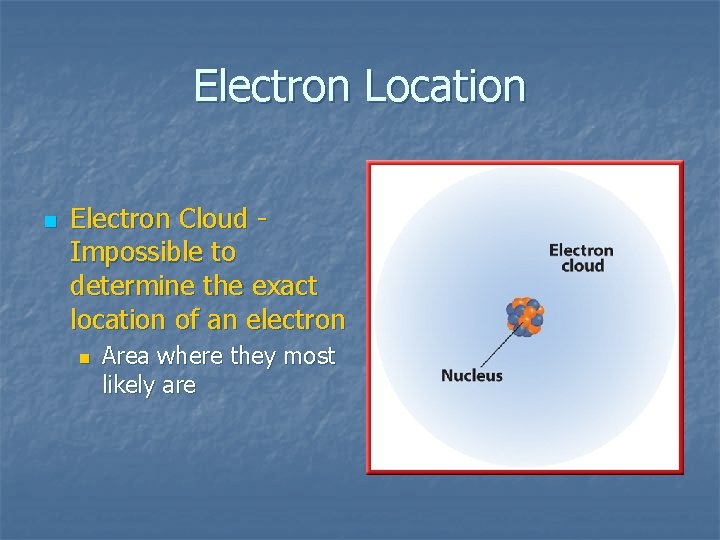
Electron Location n Electron Cloud Impossible to determine the exact location of an electron n Area where they most likely are
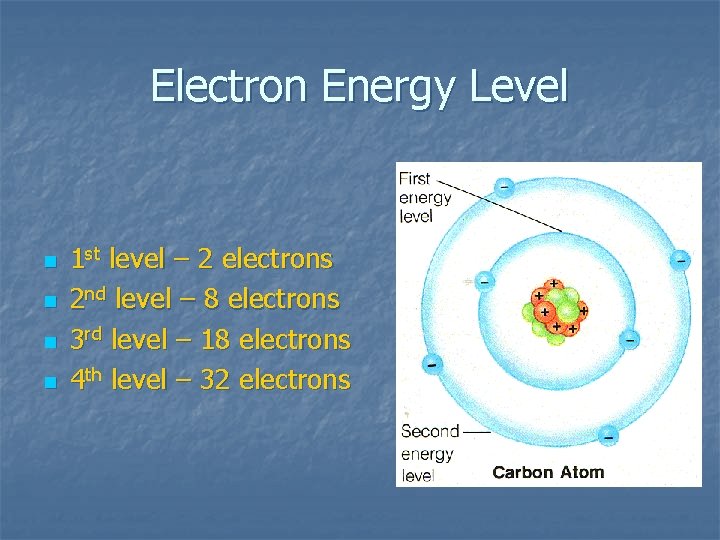
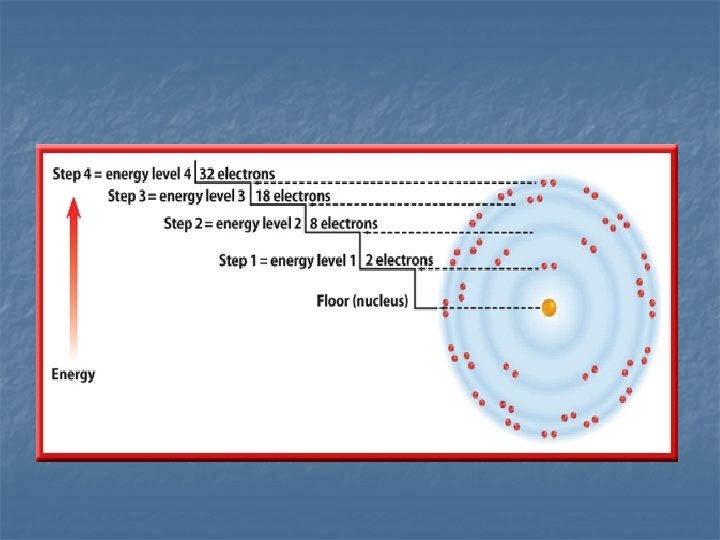
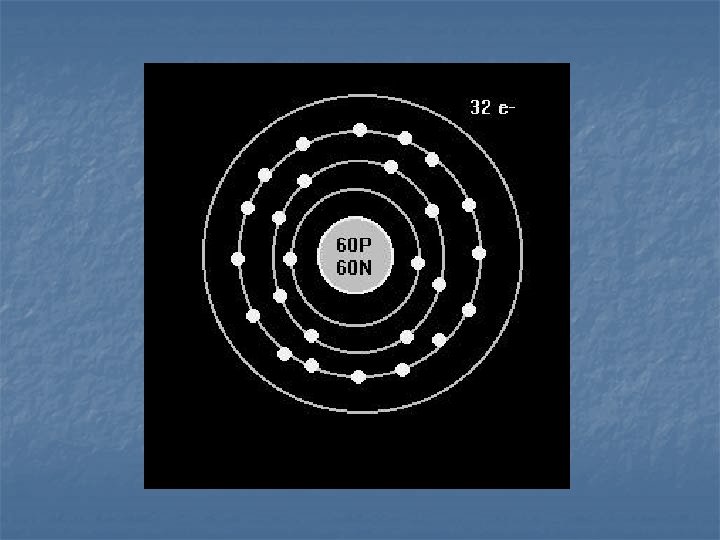
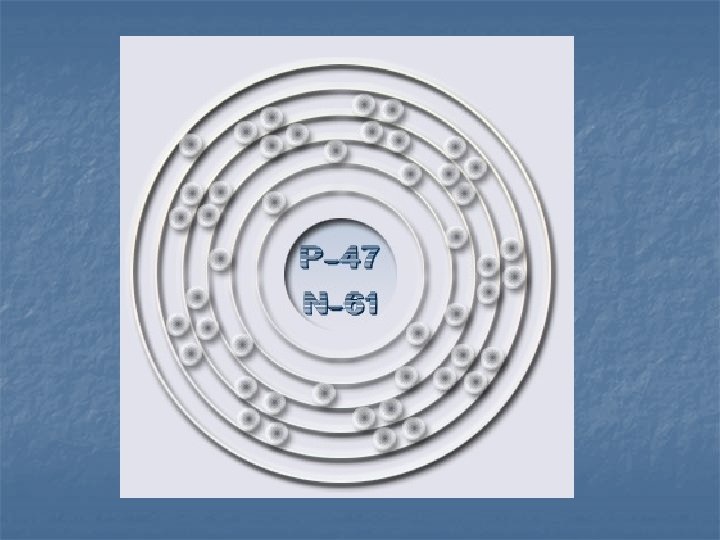
Electron Energy Level n n 1 st level – 2 electrons 2 nd level – 8 electrons 3 rd level – 18 electrons 4 th level – 32 electrons
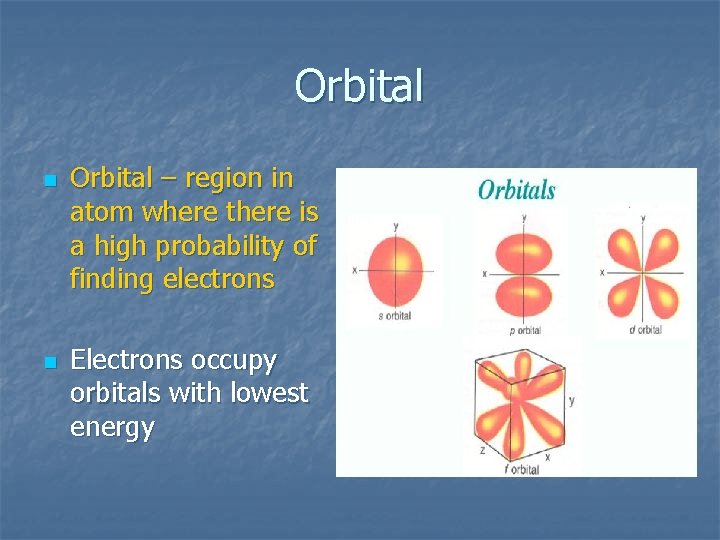
Orbital n n Orbital – region in atom where there is a high probability of finding electrons Electrons occupy orbitals with lowest energy
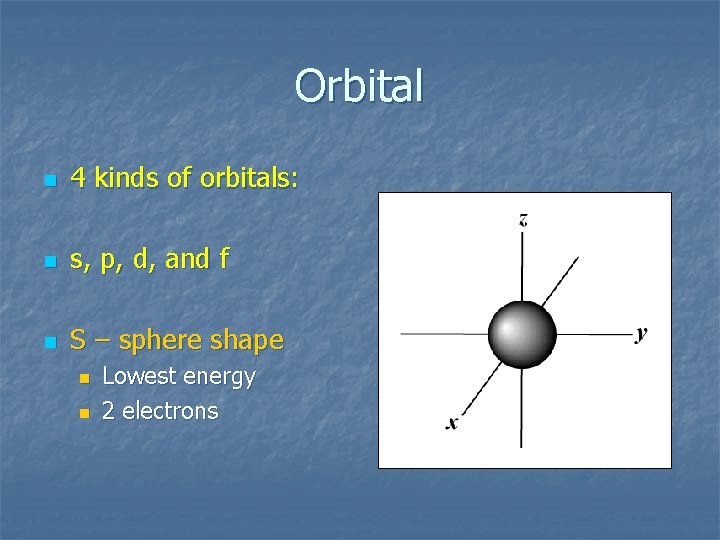
Orbital n 4 kinds of orbitals: n s, p, d, and f n S – sphere shape n n Lowest energy 2 electrons
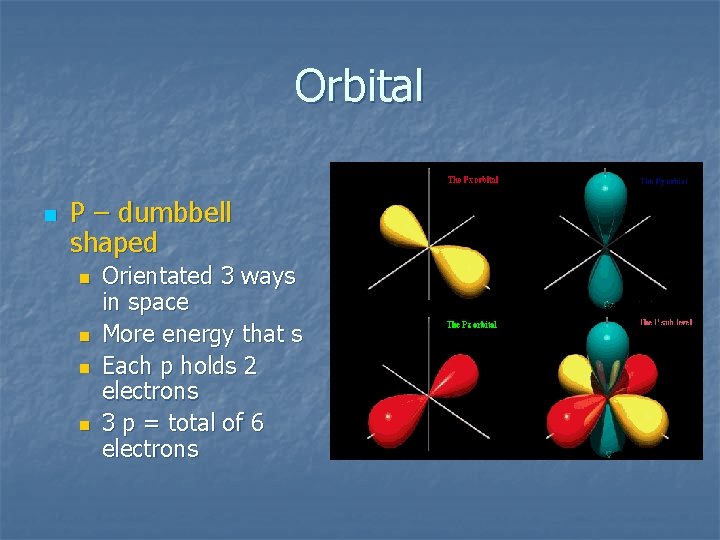
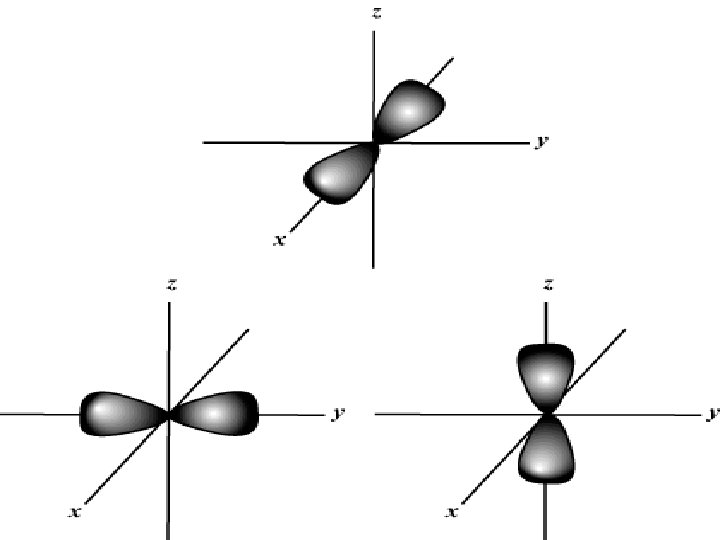
Orbital n P – dumbbell shaped n n Orientated 3 ways in space More energy that s Each p holds 2 electrons 3 p = total of 6 electrons
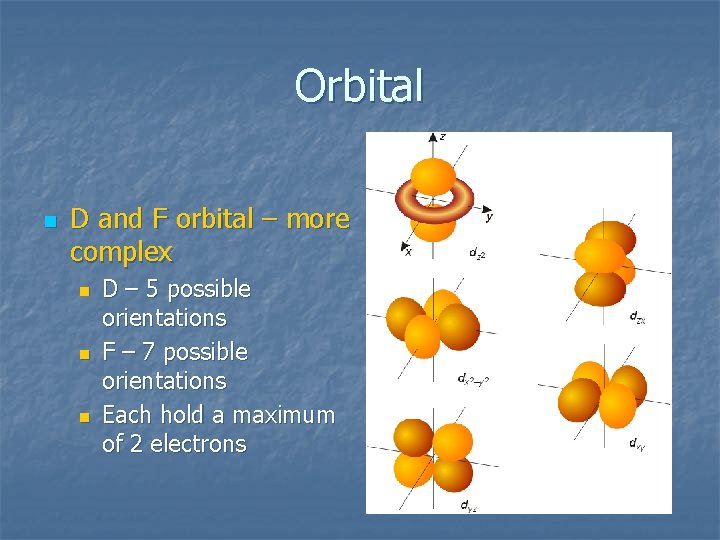
Orbital n D and F orbital – more complex n n n D – 5 possible orientations F – 7 possible orientations Each hold a maximum of 2 electrons
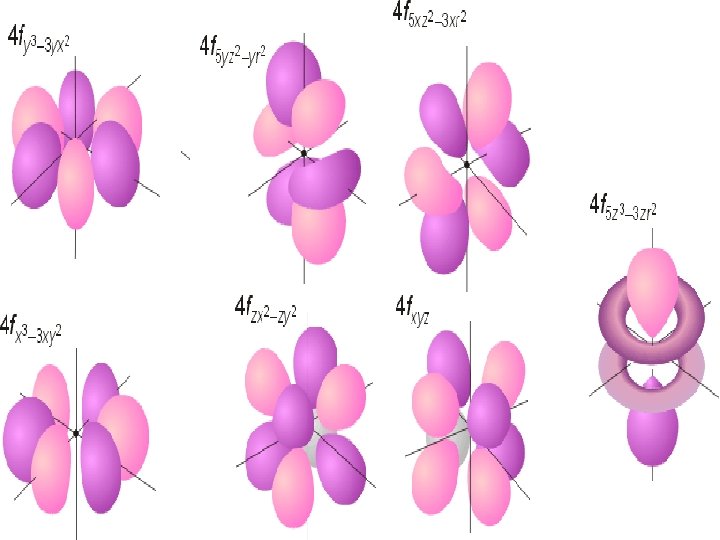
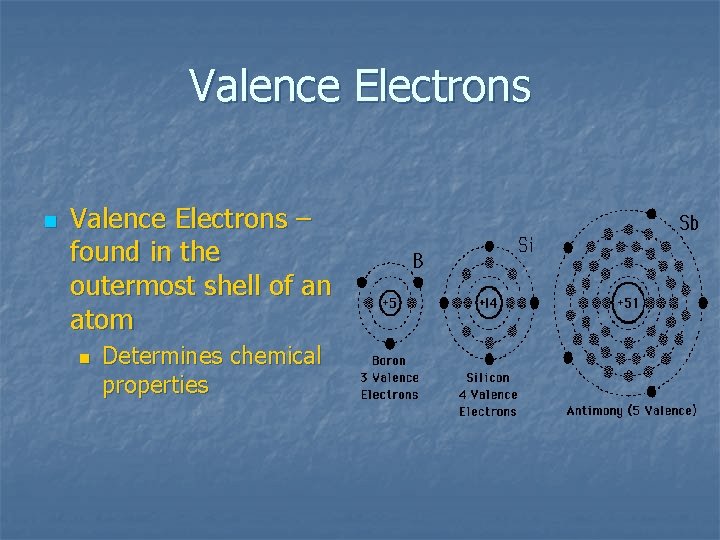
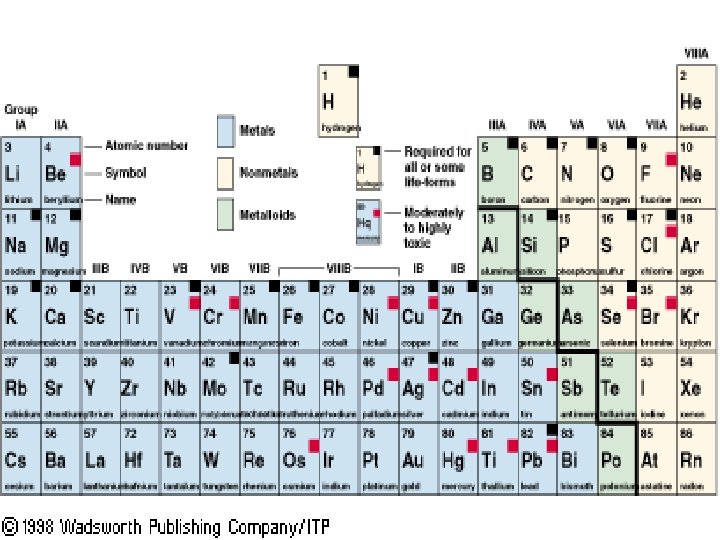
Valence Electrons n Valence Electrons – found in the outermost shell of an atom n Determines chemical properties

- Slides: 28