Chapter 30 Capillary Electrophoresis and Capillary Electrochromatography Introduction










- Slides: 17

Chapter 30 Capillary Electrophoresis and Capillary Electrochromatography

Introduction. . . • Electrophoresis is a separation method based on the differential rate of migration of charged species in a buffer solution across which has been applied a dc electric field. This separation technique was first developed by the Swedish chemist Arne Tiselius in the 1930 s for the study of serum proteins; he was awarded the 1948 Nobel Prize for this work.

• Electrophoresis has been applied to a variety of difficult analytical separation problems: inorganic anions and cations, amino acids, catecholamines, drugs, nucleic acids, nucleotides, polynucleotides, and numerous other. A particular strength of electrophoresis is its unique ability to separate charged macromolecules of interest in the biotechnology industry and in biochemical and biological research.

Types. . . Electrophoretic separations are currently performed in two quite different formats: (1) slab electrophoresis (2) capillary electrophoresis.

Slab separations. . . • Slab separations are carried out on a thin flat layer or slab of a porous semisolid gel containing an aqueous buffer solution within its pores. Ordinarily this slab has dimensions of a few centimeters on a side and, like a chromatographic thin layer plate, is capable of separating several samples simultaneously.

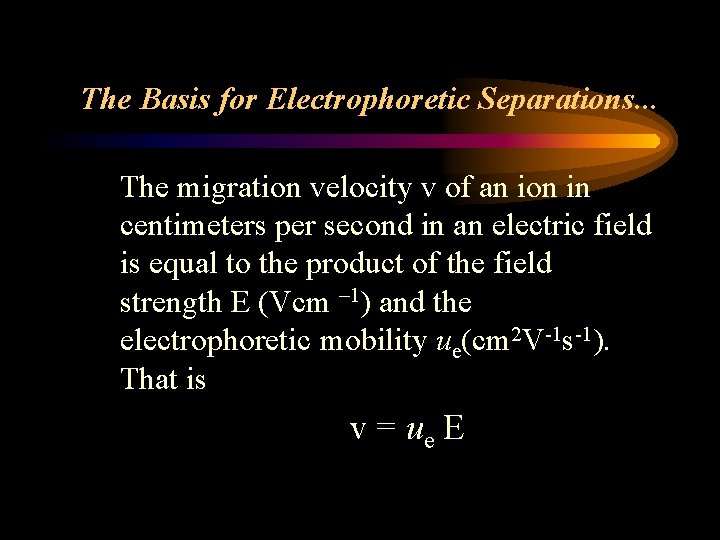
The Basis for Electrophoretic Separations. . . The migration velocity v of an ion in centimeters per second in an electric field is equal to the product of the field strength E (Vcm – 1) and the electrophoretic mobility ue(cm 2 V-1 s-1). That is v = ue E

Migration Rates and Plate Heights in Capillary Electrophoresis As we have already seen in the previous equation, an ion’s migration velocity v depends upon the electric field strength. The electric field in turn is determined by the magnitude of the applied potential (V, in volts) and the length L over which it is applied. Thus, v = ue (V/L)

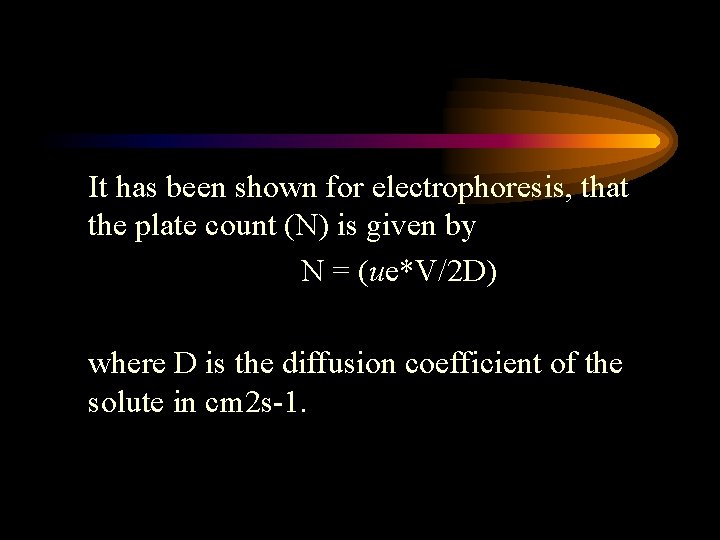
It has been shown for electrophoresis, that the plate count (N) is given by N = (ue*V/2 D) where D is the diffusion coefficient of the solute in cm 2 s-1.


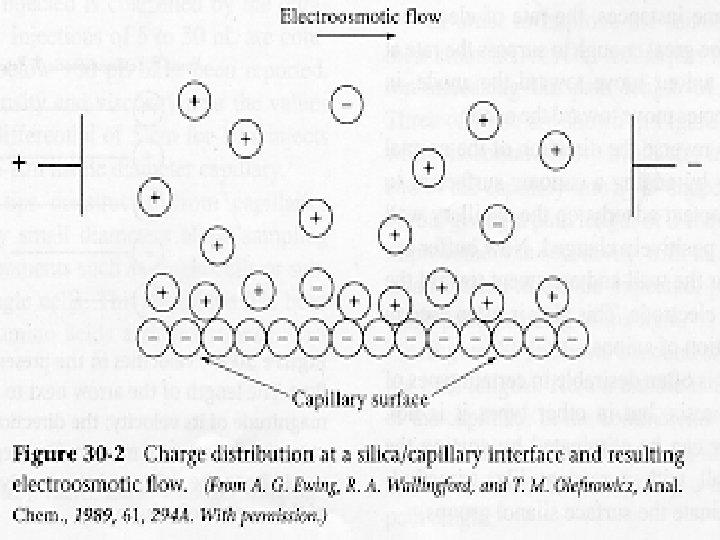
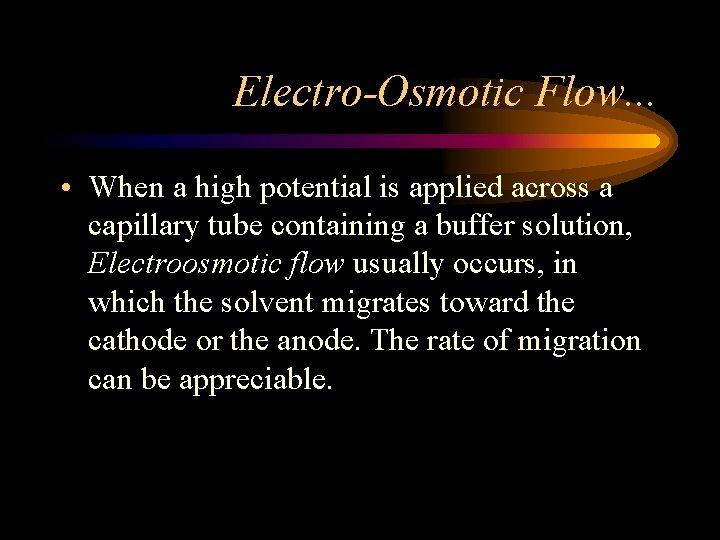
Electro-Osmotic Flow. . . • When a high potential is applied across a capillary tube containing a buffer solution, Electroosmotic flow usually occurs, in which the solvent migrates toward the cathode or the anode. The rate of migration can be appreciable.

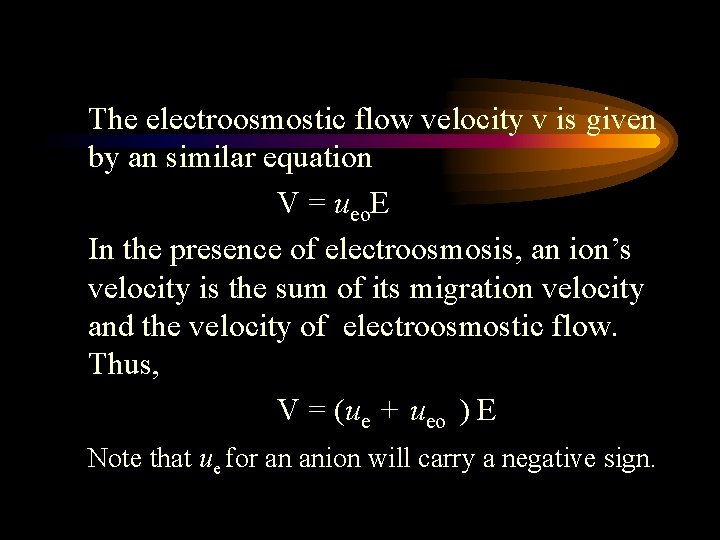
The electroosmostic flow velocity v is given by an similar equation V = ueo. E In the presence of electroosmosis, an ion’s velocity is the sum of its migration velocity and the velocity of electroosmostic flow. Thus, V = (ue + ueo ) E Note that ue for an anion will carry a negative sign.

Fluorescence Detection. . . • Just as in HPLC, fluorescence detection yields increased sensitivity and selectivity for fluorescent analytes or fluorescent derivatives. Laser-based instrumentation is preferred in order to focus the excitation radiation on the small capillary and to achieve the low detection limits available from intense source. Laser fluorescence detection has allowed detection of only 10 zeptomoles or 6000 molecules.

Electrochemical Detection. . . • Two types of electrochemical detection have been used with capillary electrophoresis: conductivity and amperometry. One of the problems with electrochemical detection has been that of isolation the detector electrodes from the high potential required for the separation.

Mass Spectrometric Detection. . . • The very small volumetric flow rates of under 1 u. L. min from electrophoresis capillaries makes it feasible to couple the effluent from the capillary of an electrophoretic device directly to the ionization source of a mass spectrometer.

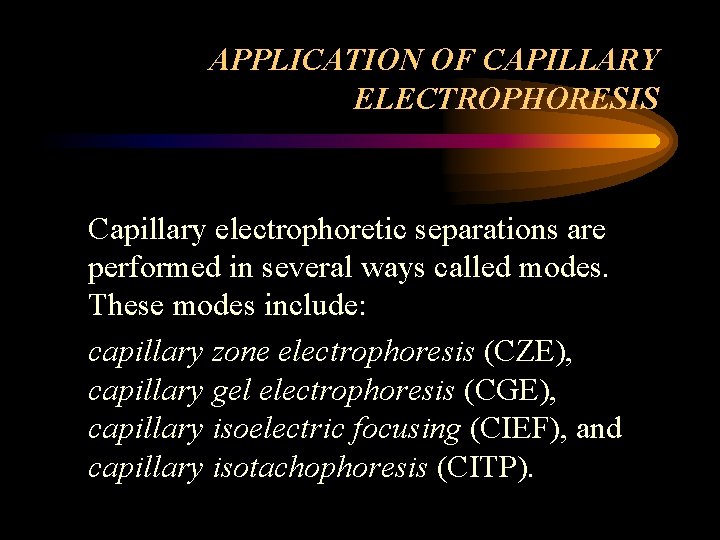
APPLICATION OF CAPILLARY ELECTROPHORESIS Capillary electrophoretic separations are performed in several ways called modes. These modes include: capillary zone electrophoresis (CZE), capillary gel electrophoresis (CGE), capillary isoelectric focusing (CIEF), and capillary isotachophoresis (CITP).

CAPILLARY ELECTROCHROMATOGRAPHY • Electrochromatography is a hybrid of capillary electrophoresis and HPLC that offers some of the best features of the two techniques. Two types of capillary electrochromatography have been developed since the early 1980 s: packed column and micellar electrokinetic capillary

References. . . • • • http: //www. acs. org http: //www. cas. org http: //www. chemcenter/org http: //www. sciencemag. org http: //www. kerouac. pharm. uky. edu/asrg/wa ve/wavehp. html