Chapter 3 Types of Matter 4 Types of

















- Slides: 27

Chapter 3 Types of Matter

4 Types of Matter


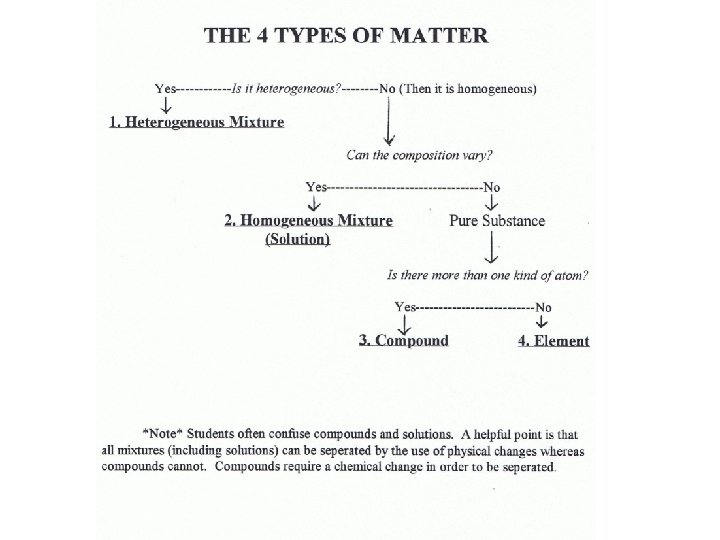
Heterogeneous vs Homogeneous • Heterogeneous matter has more than one phase (it is not uniform throughout). • Homogeneous matter has only one phase (it is uniform throughout).

Make Your Choice

Heterogeneous or Homogeneous? Ice Water

Is the matter heterogeneous? If yes. It is a Heterogeneous Mixtures What if the matter is not heterogeneous?

It is then homogenous and is either an element, compound or solution.

Homogeneous Materials Elements Compounds Solutions

If the matter is homogenous it may be a pure substance or a mixture. • Ask…Can the composition vary? Can the composition of water vary?

Can the Composition Vary? Water?

Can the Composition Vary? Is it a recipe or is it a formula?

If the composition of a homogeneous material can vary it is a solution. • A homogeneous mixture is a solution.

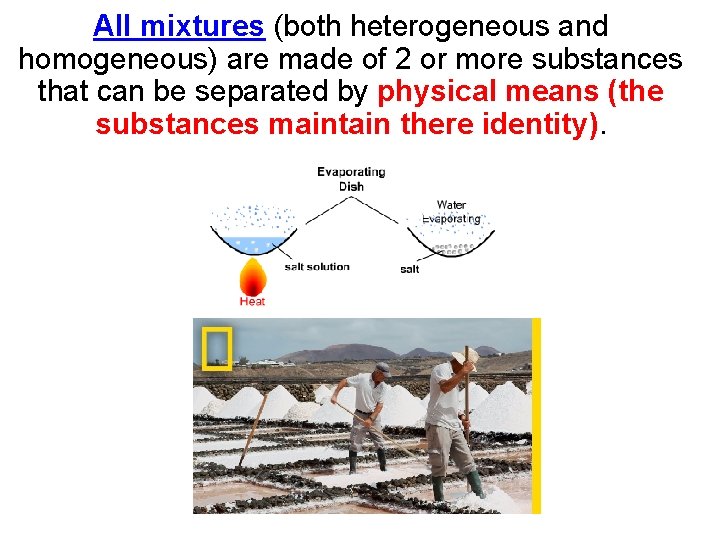
All mixtures (both heterogeneous and homogeneous) are made of 2 or more substances that can be separated by physical means (the substances maintain there identity).

Homogeneous Mixtures (Solutions)

An alloy is a homogeneous mixture of metals. • Brass is a solution of the metals copper and zinc. It is a type of alloy. An alloy is a solution of metals. • The proportions of copper and zinc can be varied to create different types of brass with varying properties. However copper is almost always the main metal present.

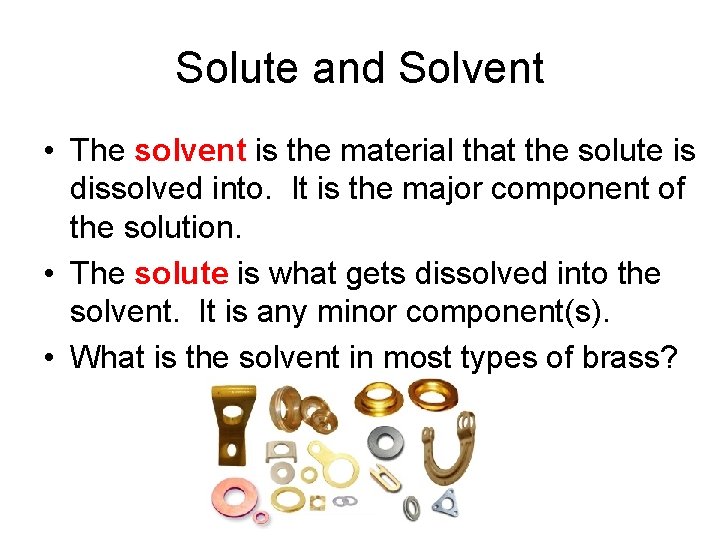
Solutions contain a solute and a solvent.

Solute and Solvent • The solvent is the material that the solute is dissolved into. It is the major component of the solution. • The solute is what gets dissolved into the solvent. It is any minor component(s). • What is the solvent in most types of brass?

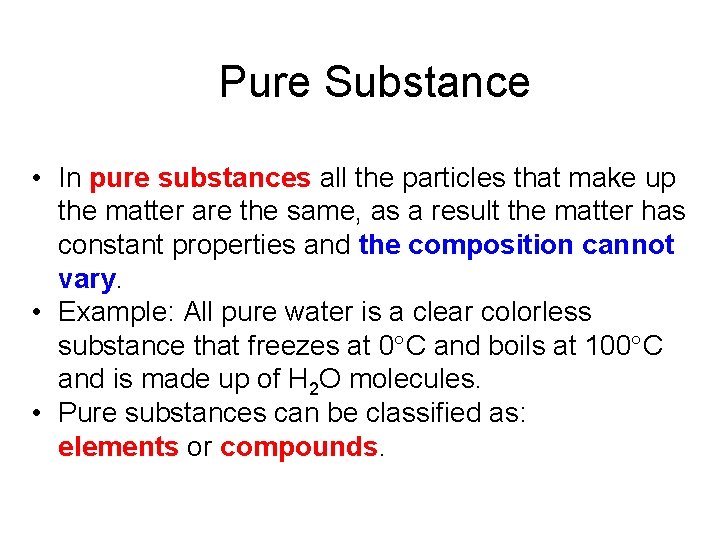
Pure Substance • In pure substances all the particles that make up the matter are the same, as a result the matter has constant properties and the composition cannot vary. • Example: All pure water is a clear colorless substance that freezes at 0 C and boils at 100 C and is made up of H 2 O molecules. • Pure substances can be classified as: elements or compounds.

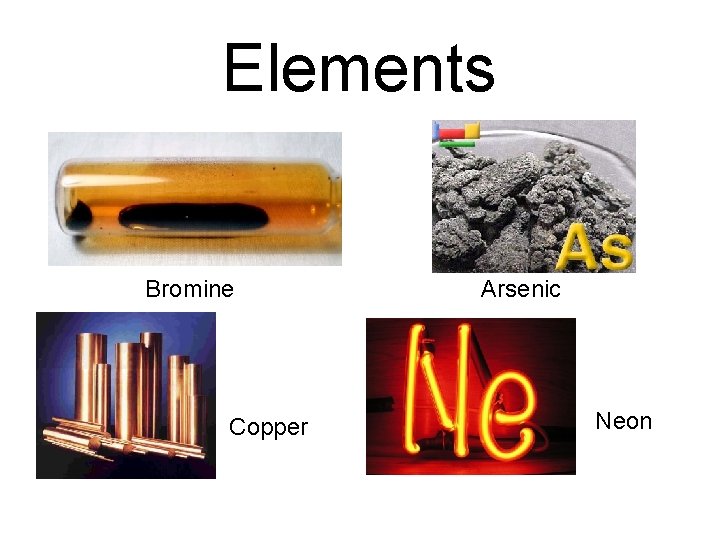
Elements Bromine Copper Arsenic Neon

Elements • Elements cannot be broken down into a simpler substance because they are made of only 1 kind of atom (gold, oxygen, mercury). • Elements can be found on the periodic table. • Elements can exist as atoms or molecules.

Elements • A combination of two or more atoms of the same element are considered molecules of the element not a compound. For example, diatomic molecules like O 2, H 2, N 2 or polyatomic molecules like P 4.

Compounds Nitrogen dioxide (NO 2) Sodium chloride (Na. Cl)

Compounds • Compounds contain 2 or more different elements in a fixed proportion. • Compounds are identified with “exact” chemical formulas. • Examples: CO 2, H 2 O, Na. Cl C 12 H 22 O 11. • Compounds are a chemical combination of elements. This means that the elements are bonded together and separating them breaks the bonds and creates new substances.

Compounds are a chemical combination of elements.

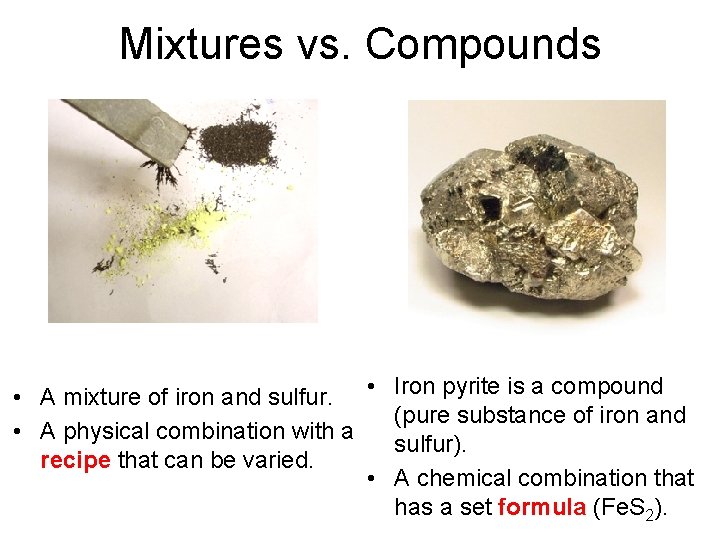
Mixtures vs. Compounds • A mixture of iron and sulfur. • • A physical combination with a recipe that can be varied. • Iron pyrite is a compound (pure substance of iron and sulfur). A chemical combination that has a set formula (Fe. S 2).

Homework • Worksheet: Classification of Matter (due in 2 days).