Chapter 3 Topics Molecular Formulas Ionic Compounds Ions


- Slides: 25

Chapter 3 Topics • Molecular Formulas • Ionic Compounds – Ions and charges – Naming ionic compounds • Molecular Compounds • The Mole – Mole to mass; mass to mole • Describing Compound Formulas – Mass percent – percent composition – Empirical and Molecular Formulas 11/9/2020 Kull Chem 105 Chapter 2 1

Molecular Formulas • Molecule: smallest identifiable unit of pure substance; still maintains composition and chemical properties – Formula (molecular): description of the composition C 3 H 6 O 2 – Condensed formula CH 3 COCH 2 OH CH 3 OCH 2 CHO – Structural formula 11/9/2020 2

Ionic Compounds • Ions and charges • Naming ionic compounds 11/9/2020 3

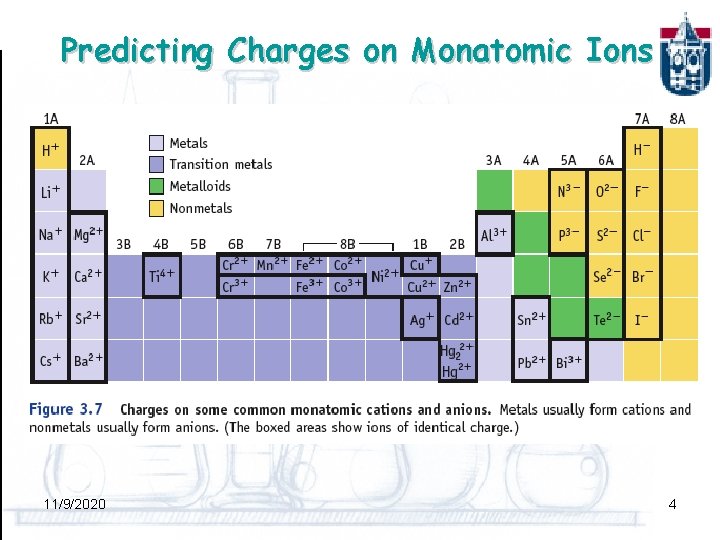
Predicting Charges on Monatomic Ions 11/9/2020 4

3. 4 Molecular Compounds • • Nonmetal to nonmetal Use prefixes – mono, di, tri, etc. Second component add –ide Only one element in cation spot, mono not required Phosporus triiodide N 2 F 4 Dioxygen difluoride P 4 O 10 11/9/2020 5

3. 5 The Mole to mass; mass to mole • • Citric acid C 6 H 8 O 7 Mg. CO 3 Mg. SO 4· 7 H 2 O Formula mass Mass percent Moles to grams Grams to molecules % composition 11/9/2020 6

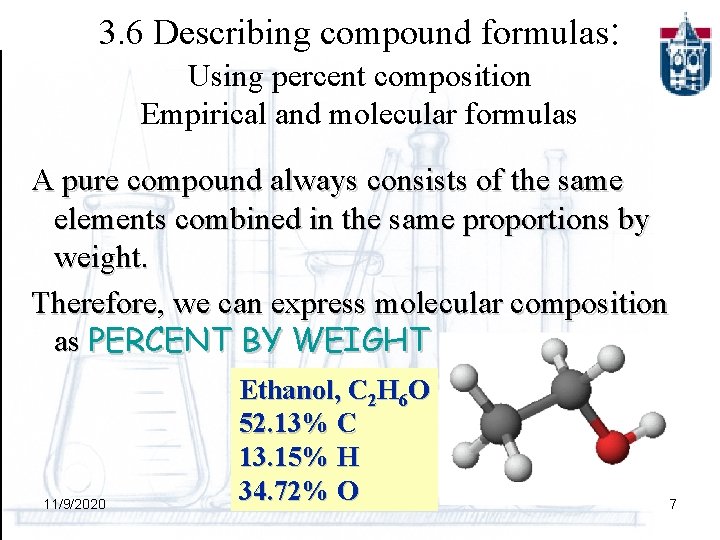
3. 6 Describing compound formulas: Using percent composition Empirical and molecular formulas A pure compound always consists of the same elements combined in the same proportions by weight. Therefore, we can express molecular composition as PERCENT BY WEIGHT 11/9/2020 Ethanol, C 2 H 6 O 52. 13% C 13. 15% H 34. 72% O 7

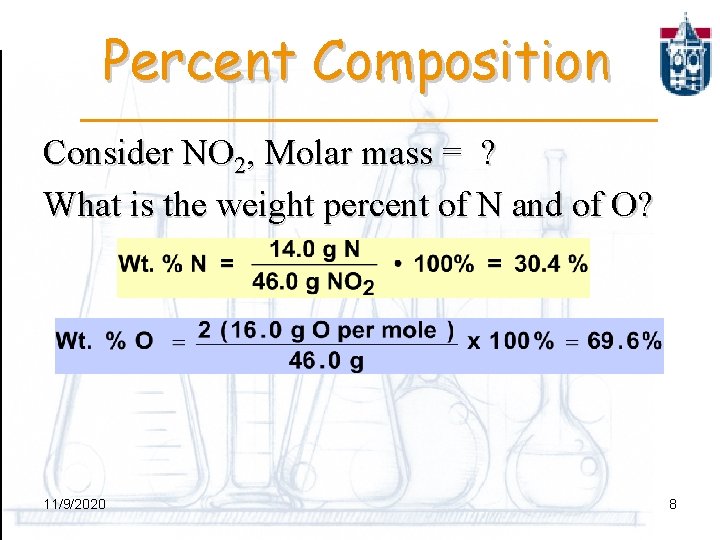
Percent Composition Consider NO 2, Molar mass = ? What is the weight percent of N and of O? 11/9/2020 8

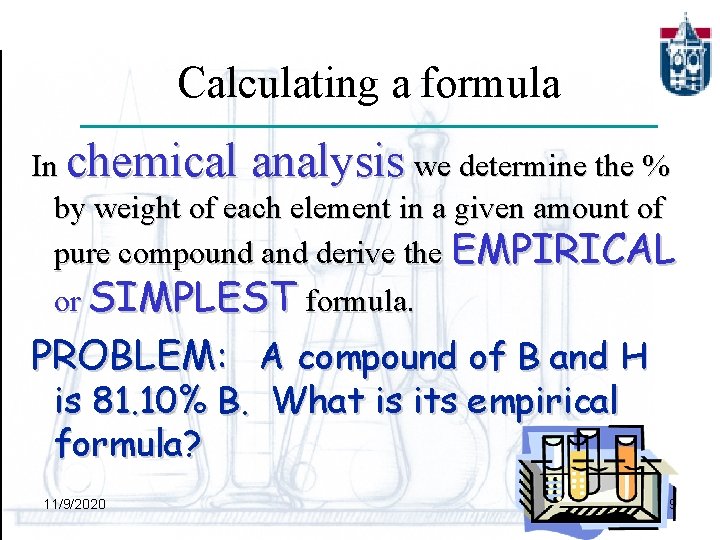
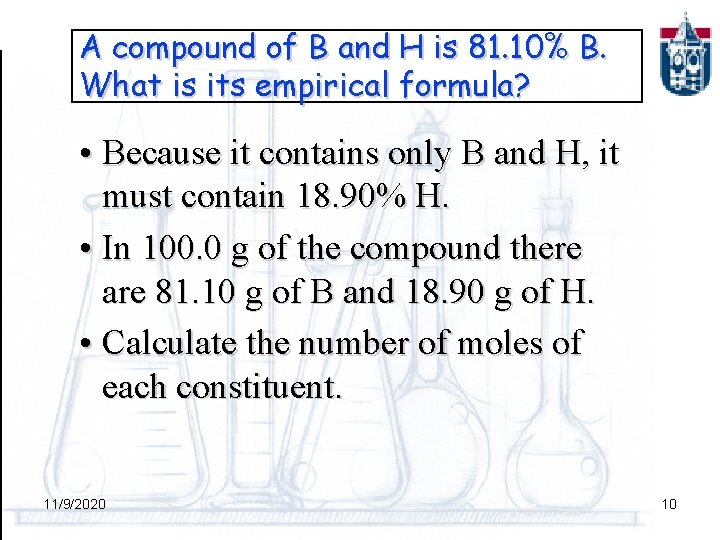
Calculating a formula In chemical analysis we determine the % by weight of each element in a given amount of pure compound and derive the EMPIRICAL or SIMPLEST formula. PROBLEM: A compound of B and H is 81. 10% B. What is its empirical formula? 11/9/2020 9

A compound of B and H is 81. 10% B. What is its empirical formula? • Because it contains only B and H, it must contain 18. 90% H. • In 100. 0 g of the compound there are 81. 10 g of B and 18. 90 g of H. • Calculate the number of moles of each constituent. 11/9/2020 10

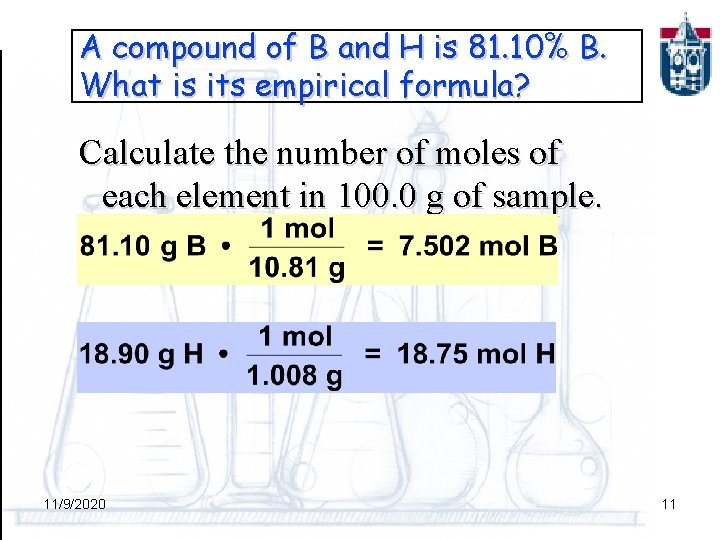
A compound of B and H is 81. 10% B. What is its empirical formula? Calculate the number of moles of each element in 100. 0 g of sample. 11/9/2020 11

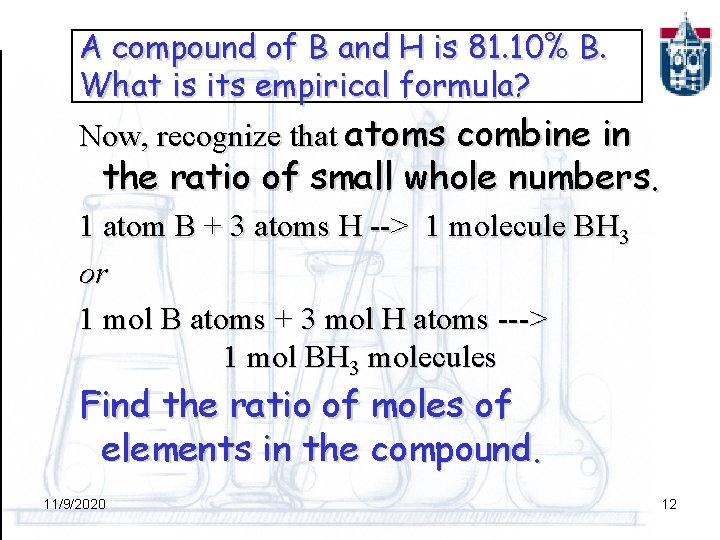
A compound of B and H is 81. 10% B. What is its empirical formula? Now, recognize that atoms combine in the ratio of small whole numbers. 1 atom B + 3 atoms H --> 1 molecule BH 3 or 1 mol B atoms + 3 mol H atoms ---> 1 mol BH 3 molecules Find the ratio of moles of elements in the compound. 11/9/2020 12

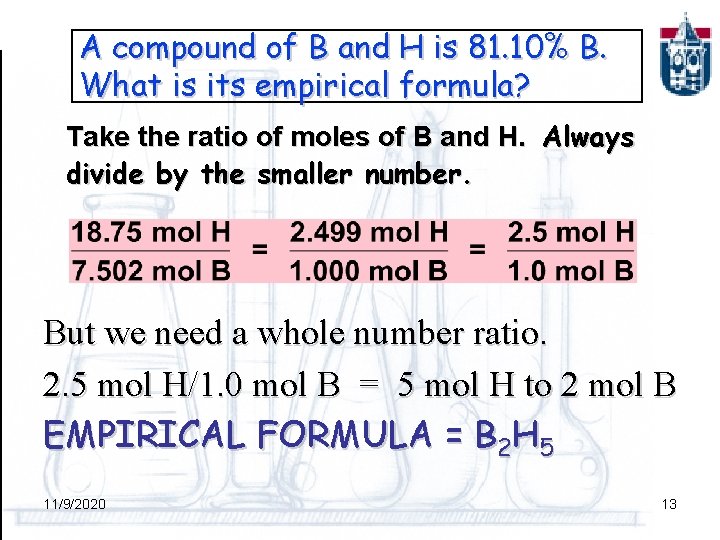
A compound of B and H is 81. 10% B. What is its empirical formula? Take the ratio of moles of B and H. Always divide by the smaller number. But we need a whole number ratio. 2. 5 mol H/1. 0 mol B = 5 mol H to 2 mol B EMPIRICAL FORMULA = B 2 H 5 11/9/2020 13

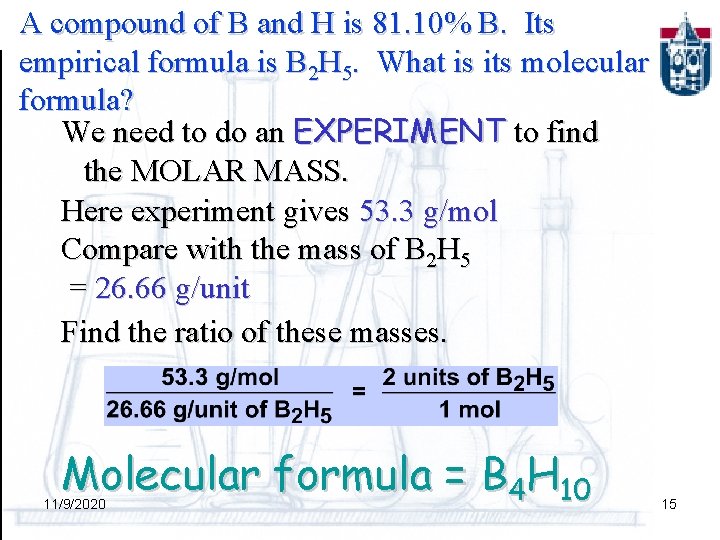
A compound of B and H is 81. 10% B. Its empirical formula is B 2 H 5. What is its molecular formula? Is the molecular formula B 2 H 5, B 4 H 10, B 6 H 15, B 8 H 20, etc. ? B 2 H 6 is one example of this class of compounds. 11/9/2020 14

A compound of B and H is 81. 10% B. Its empirical formula is B 2 H 5. What is its molecular formula? We need to do an EXPERIMENT to find the MOLAR MASS. Here experiment gives 53. 3 g/mol Compare with the mass of B 2 H 5 = 26. 66 g/unit Find the ratio of these masses. Molecular formula = B 4 H 10 11/9/2020 15

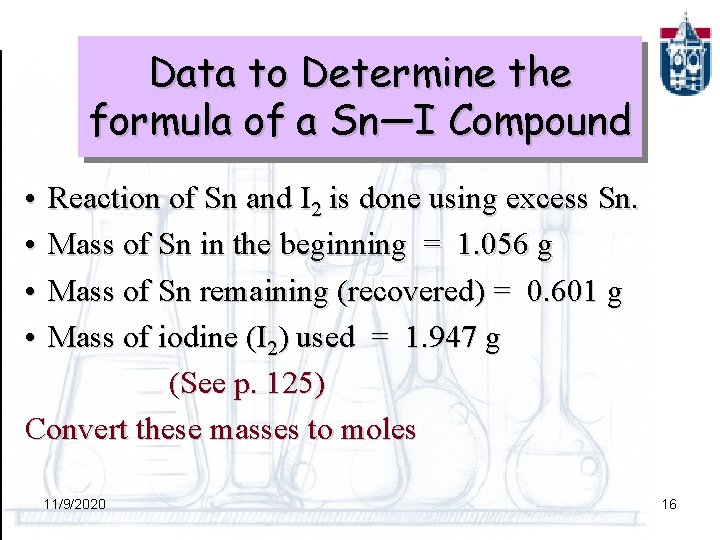
Data to Determine the formula of a Sn—I Compound • • Reaction of Sn and I 2 is done using excess Sn. Mass of Sn in the beginning = 1. 056 g Mass of Sn remaining (recovered) = 0. 601 g Mass of iodine (I 2) used = 1. 947 g (See p. 125) Convert these masses to moles 11/9/2020 16

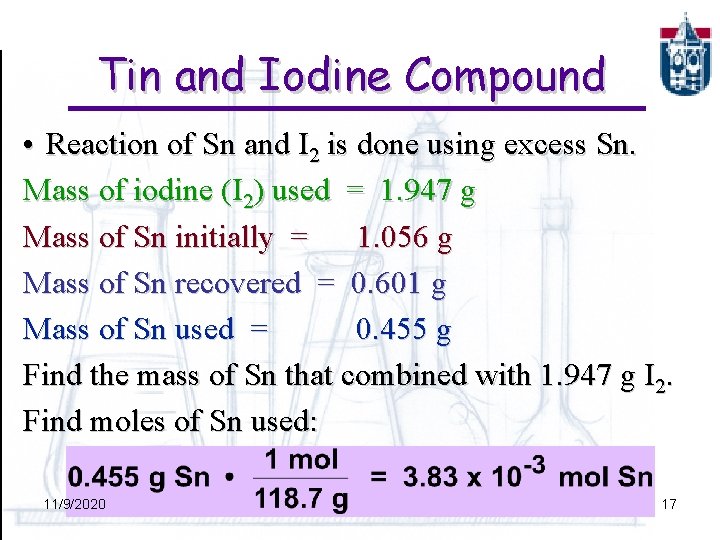
Tin and Iodine Compound • Reaction of Sn and I 2 is done using excess Sn. Mass of iodine (I 2) used = 1. 947 g Mass of Sn initially = 1. 056 g Mass of Sn recovered = 0. 601 g Mass of Sn used = 0. 455 g Find the mass of Sn that combined with 1. 947 g I 2. Find moles of Sn used: 11/9/2020 17

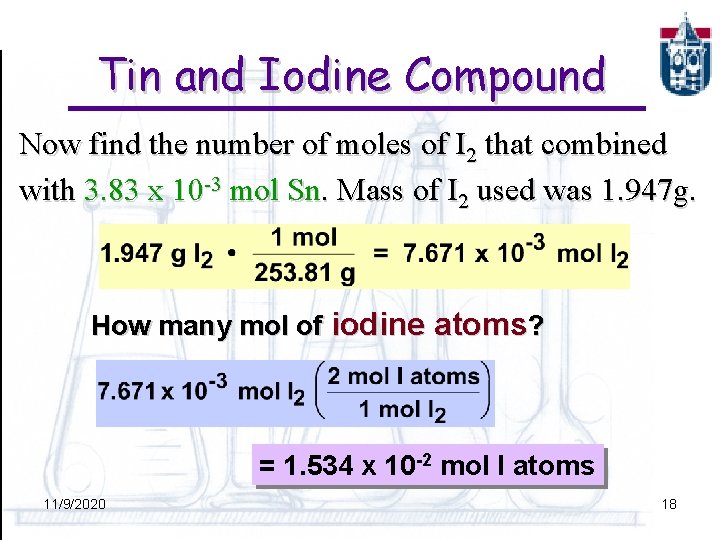
Tin and Iodine Compound Now find the number of moles of I 2 that combined with 3. 83 x 10 -3 mol Sn. Mass of I 2 used was 1. 947 g. How many mol of iodine atoms? = 1. 534 x 10 -2 mol I atoms 11/9/2020 18

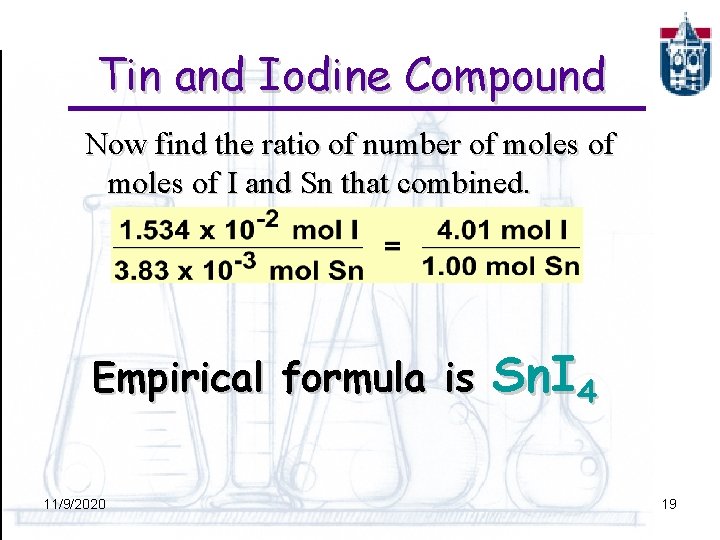
Tin and Iodine Compound Now find the ratio of number of moles of I and Sn that combined. Empirical formula is 11/9/2020 Sn. I 4 19

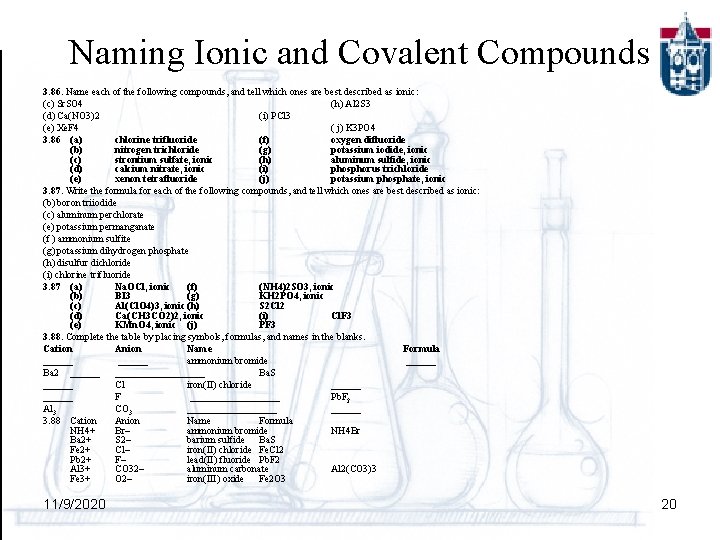
Naming Ionic and Covalent Compounds 3. 86. Name each of the following compounds, and tell which ones are best described as ionic: (c) Sr. SO 4 (h) Al 2 S 3 (d) Ca(NO 3)2 (i) PCl 3 (e) Xe. F 4 ( j) K 3 PO 4 3. 86 (a) chlorine trifluoride (f) oxygen difluoride (b) nitrogen trichloride (g) potassium iodide, ionic (c) strontium sulfate, ionic (h) aluminum sulfide, ionic (d) calcium nitrate, ionic (i) phosphorus trichloride (e) xenon tetrafluoride (j) potassium phosphate, ionic 3. 87. Write the formula for each of the following compounds, and tell which ones are best described as ionic: (b) boron triiodide (c) aluminum perchlorate (e) potassium permanganate (f ) ammonium sulfite (g) potassium dihydrogen phosphate (h) disulfur dichloride (i) chlorine trifluoride 3. 87 (a) Na. OCl, ionic (f) (NH 4)2 SO 3, ionic (b) BI 3 (g) KH 2 PO 4, ionic (c) Al(Cl. O 4)3, ionic (h) S 2 Cl 2 (d) Ca(CH 3 CO 2)2, ionic (i) Cl. F 3 (e) KMn. O 4, ionic (j) PF 3 3. 88. Complete the table by placing symbols, formulas, and names in the blanks. Cation Anion Name Formula ______ ammonium bromide ______ Ba 2 ____________ Ba. S ______ Cl iron(II) chloride ______ F _________ Pb. F 2 Al 3 CO 3 _________ 3. 88 Cation Anion Name Formula NH 4+ Br– ammonium bromide NH 4 Br Ba 2+ S 2– barium sulfide Ba. S Fe 2+ Cl– iron(II) chloride Fe. Cl 2 Pb 2+ F– lead(II) fluoride Pb. F 2 Al 3+ CO 32– aluminum carbonate Al 2(CO 3)3 Fe 3+ O 2– iron(III) oxide Fe 2 O 3 11/9/2020 20

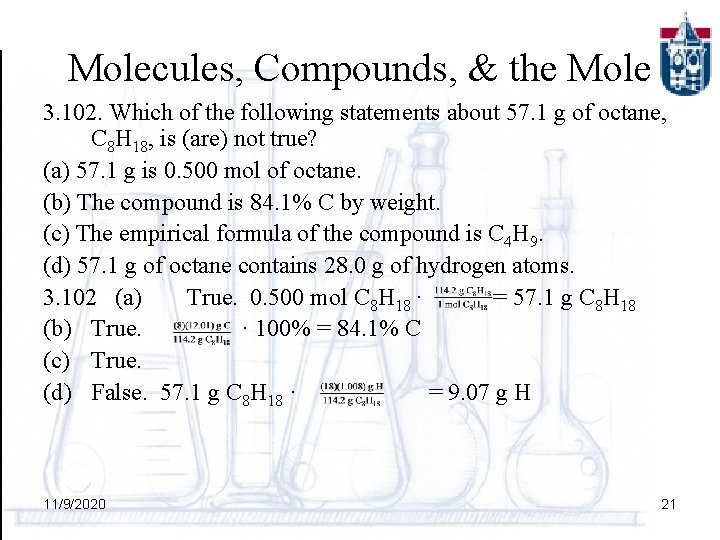
Molecules, Compounds, & the Mole 3. 102. Which of the following statements about 57. 1 g of octane, C 8 H 18, is (are) not true? (a) 57. 1 g is 0. 500 mol of octane. (b) The compound is 84. 1% C by weight. (c) The empirical formula of the compound is C 4 H 9. (d) 57. 1 g of octane contains 28. 0 g of hydrogen atoms. 3. 102 (a) True. 0. 500 mol C 8 H 18 · = 57. 1 g C 8 H 18 (b) True. · 100% = 84. 1% C (c) True. (d) False. 57. 1 g C 8 H 18 · = 9. 07 g H 11/9/2020 21

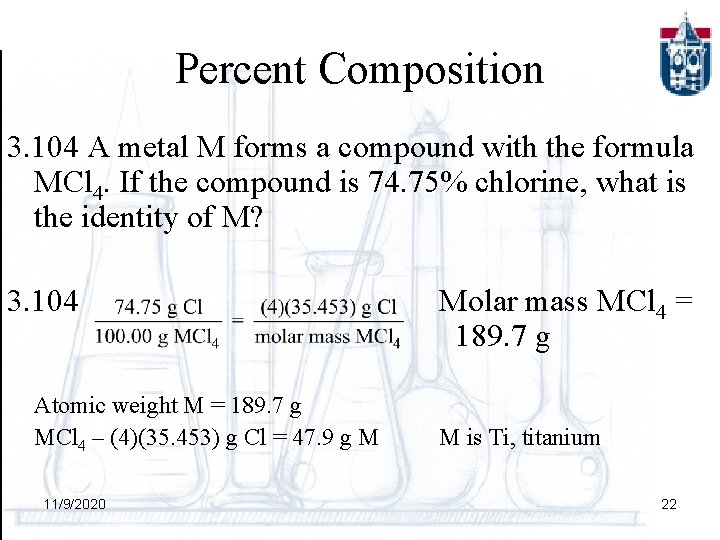
Percent Composition 3. 104 A metal M forms a compound with the formula MCl 4. If the compound is 74. 75% chlorine, what is the identity of M? 3. 104 Atomic weight M = 189. 7 g MCl 4 – (4)(35. 453) g Cl = 47. 9 g M 11/9/2020 Molar mass MCl 4 = 189. 7 g M is Ti, titanium 22

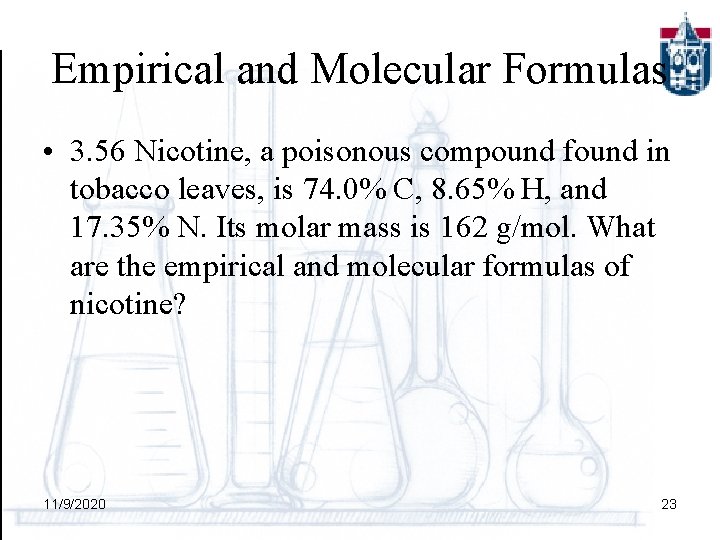
Empirical and Molecular Formulas • 3. 56 Nicotine, a poisonous compound found in tobacco leaves, is 74. 0% C, 8. 65% H, and 17. 35% N. Its molar mass is 162 g/mol. What are the empirical and molecular formulas of nicotine? 11/9/2020 23

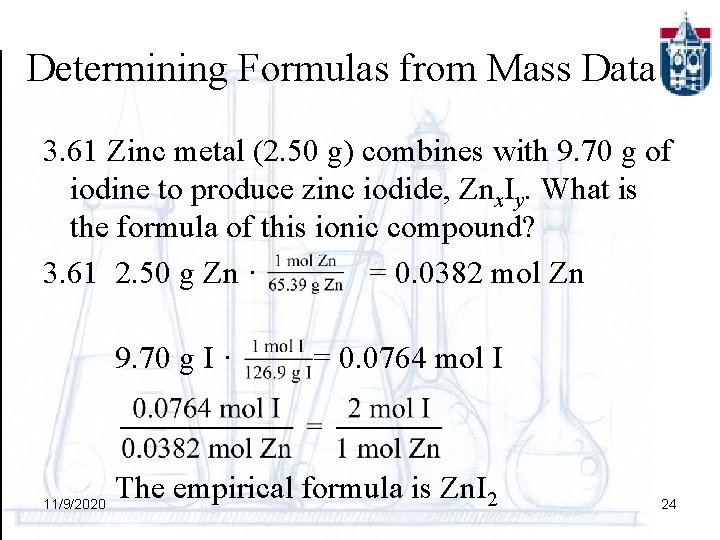
Determining Formulas from Mass Data 3. 61 Zinc metal (2. 50 g) combines with 9. 70 g of iodine to produce zinc iodide, Znx. Iy. What is the formula of this ionic compound? 3. 61 2. 50 g Zn · = 0. 0382 mol Zn 9. 70 g I · = 0. 0764 mol I 11/9/2020 The empirical formula is Zn. I 2 24

3. 63. Write formulas for all of the compounds that can be made by combining the cations NH 4 and Ni 2 with the anions CO 3 and SO 4. 3. 63 (NH 4)2 CO 3 (NH 4)2 SO 4 Ni. CO 3 Ni. SO 4 11/9/2020 25