Chapter 3 The Physical Science of the Environment











- Slides: 77

Chapter 3 – The Physical Science of the Environment

Chapter 3 Outline • Chemistry – Atoms and Elements – Water – Organic Molecules • Energy – Laws of Thermodynamics – Forms of Energy • Earth Science – Structure of Earth – Lithosphere – Atmosphere • Sun – Source of Energy • Weather and Climate

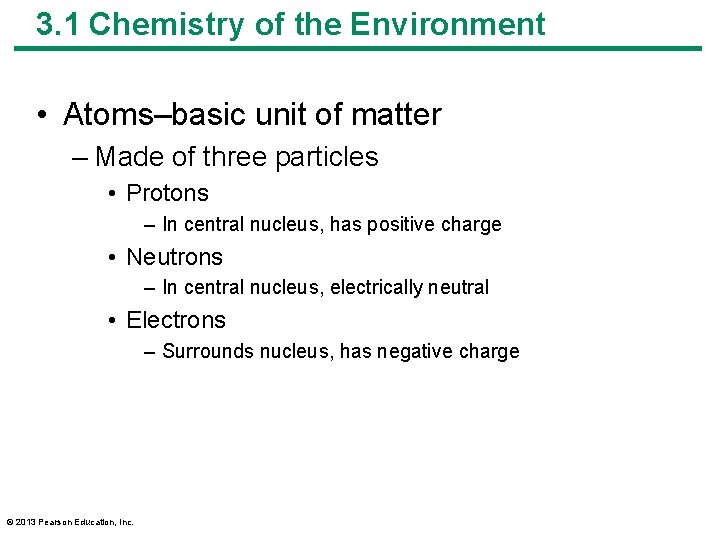
3. 1 Chemistry of the Environment • Atoms–basic unit of matter – Made of three particles • Protons – In central nucleus, has positive charge • Neutrons – In central nucleus, electrically neutral • Electrons – Surrounds nucleus, has negative charge © 2013 Pearson Education, Inc.

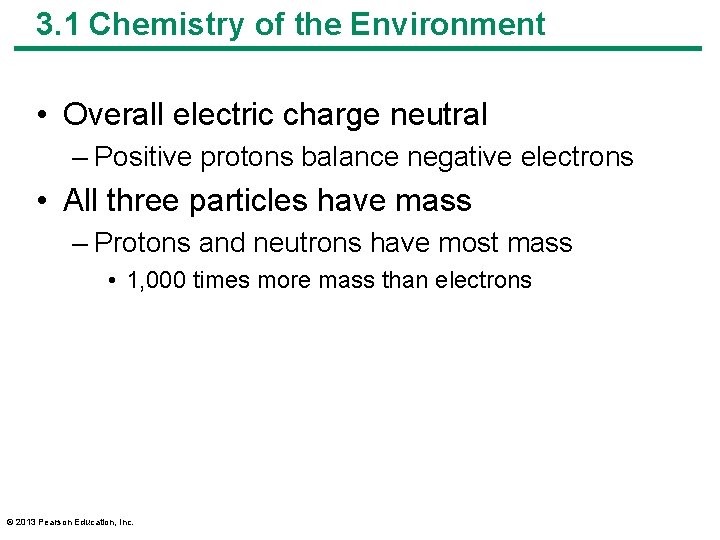
3. 1 Chemistry of the Environment • Overall electric charge neutral – Positive protons balance negative electrons • All three particles have mass – Protons and neutrons have most mass • 1, 000 times more mass than electrons © 2013 Pearson Education, Inc.

© 2013 Pearson Education, Inc.

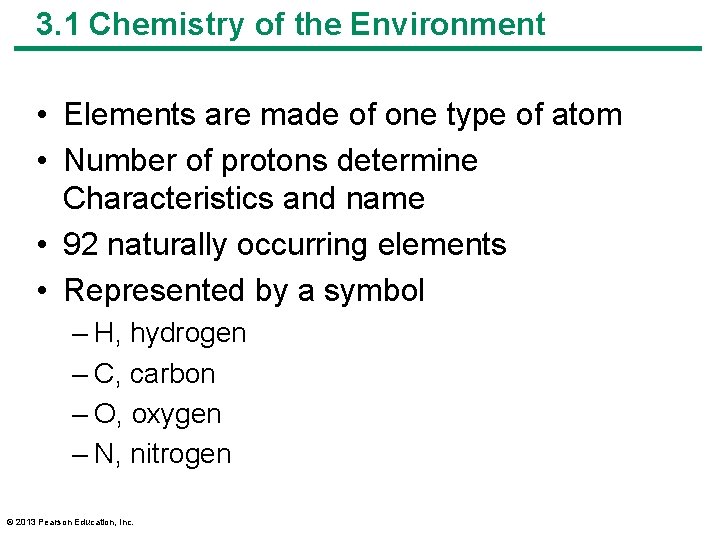
3. 1 Chemistry of the Environment • Elements are made of one type of atom • Number of protons determine Characteristics and name • 92 naturally occurring elements • Represented by a symbol – H, hydrogen – C, carbon – O, oxygen – N, nitrogen © 2013 Pearson Education, Inc.

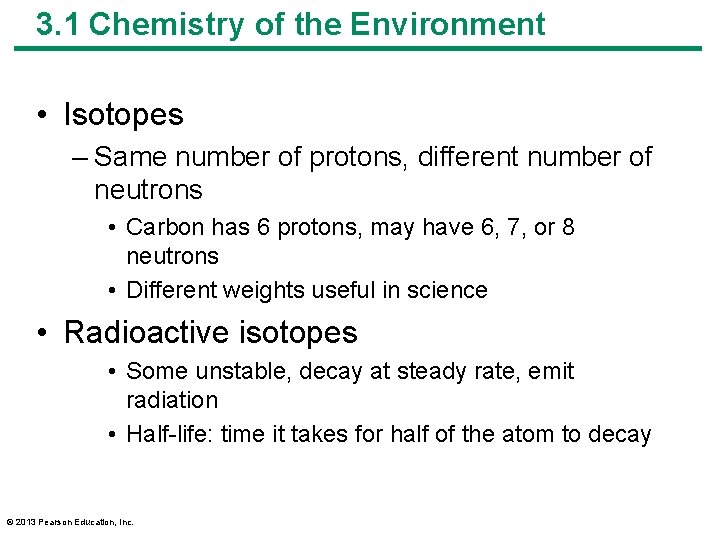
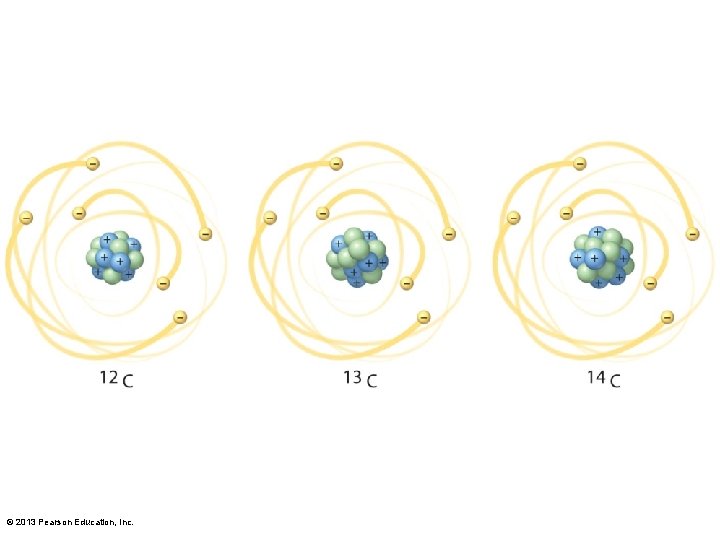
3. 1 Chemistry of the Environment • Isotopes – Same number of protons, different number of neutrons • Carbon has 6 protons, may have 6, 7, or 8 neutrons • Different weights useful in science • Radioactive isotopes • Some unstable, decay at steady rate, emit radiation • Half-life: time it takes for half of the atom to decay © 2013 Pearson Education, Inc.

© 2013 Pearson Education, Inc.

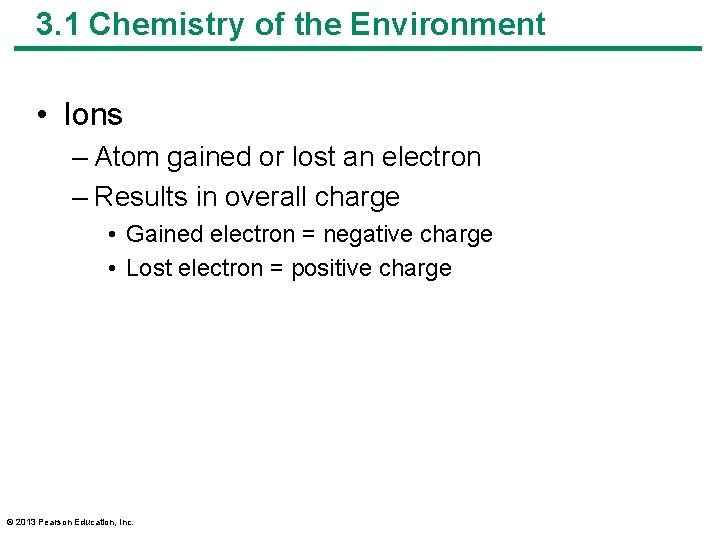
3. 1 Chemistry of the Environment • Ions – Atom gained or lost an electron – Results in overall charge • Gained electron = negative charge • Lost electron = positive charge © 2013 Pearson Education, Inc.

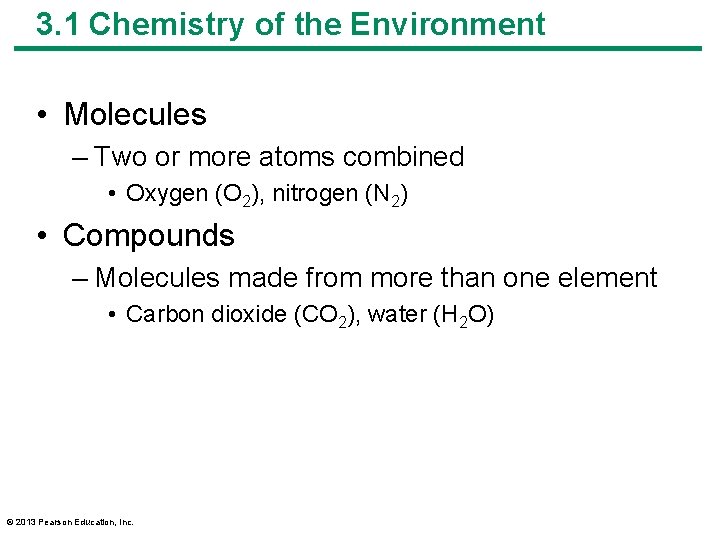
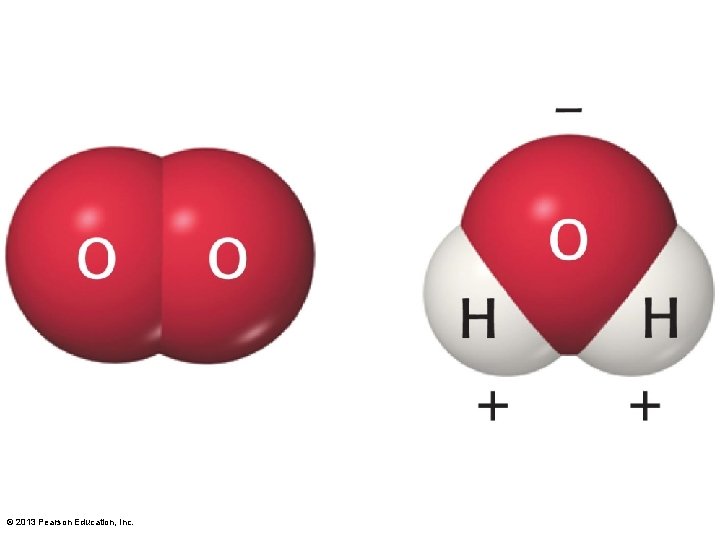
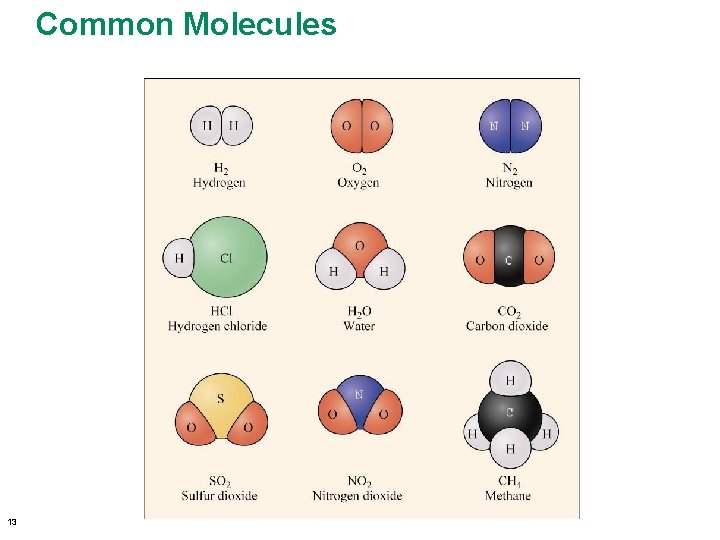
3. 1 Chemistry of the Environment • Molecules – Two or more atoms combined • Oxygen (O 2), nitrogen (N 2) • Compounds – Molecules made from more than one element • Carbon dioxide (CO 2), water (H 2 O) © 2013 Pearson Education, Inc.

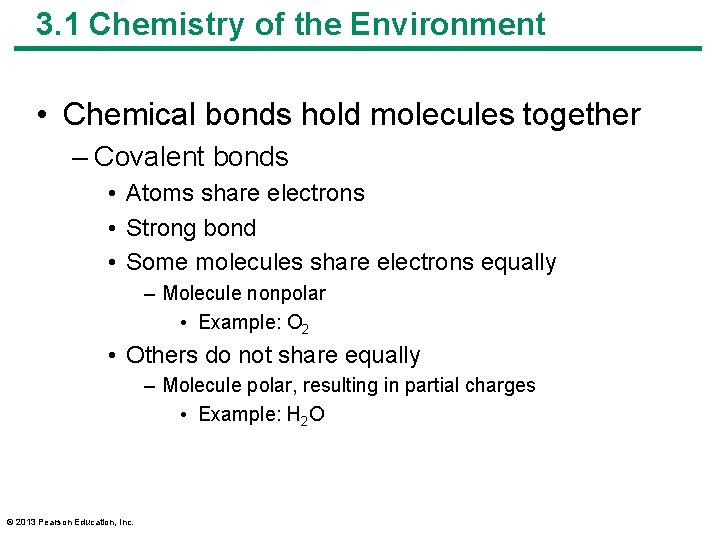
3. 1 Chemistry of the Environment • Chemical bonds hold molecules together – Covalent bonds • Atoms share electrons • Strong bond • Some molecules share electrons equally – Molecule nonpolar • Example: O 2 • Others do not share equally – Molecule polar, resulting in partial charges • Example: H 2 O © 2013 Pearson Education, Inc.

© 2013 Pearson Education, Inc.

Common Molecules 13

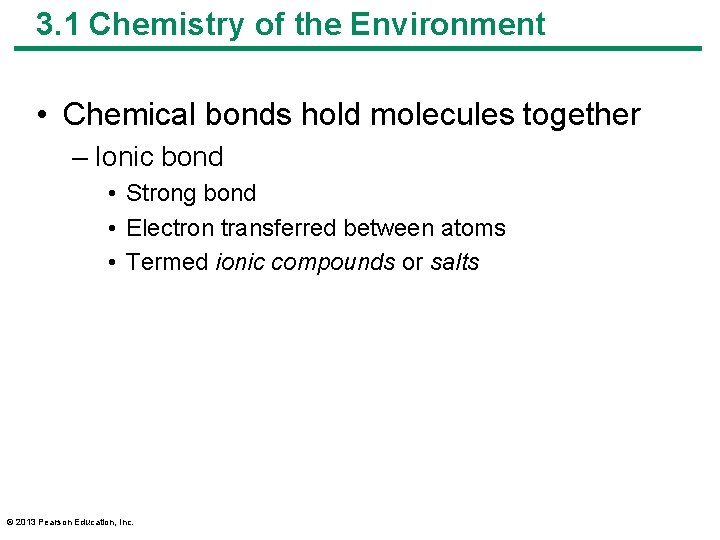
3. 1 Chemistry of the Environment • Chemical bonds hold molecules together – Ionic bond • Strong bond • Electron transferred between atoms • Termed ionic compounds or salts © 2013 Pearson Education, Inc.

3. 1 Chemistry of the Environment • Dipole bonds – Weaker bonds – Between atoms and molecules • Result of shifts of charge – Many biological functions © 2013 Pearson Education, Inc.

3. 1 Chemistry of the Environment • Water–important to life – Polar molecule • Forms dipole bonds with other water molecules – Gives water unique properties, stability • An excellent solvent – Many biological functions use water as solvent. © 2013 Pearson Education, Inc.

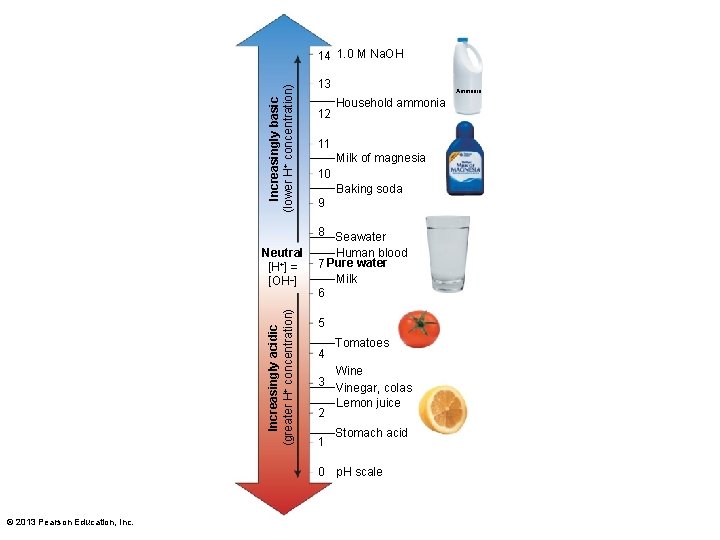
3. 1 Chemistry of the Environment • Water–important to life – Acids and bases • Water dissociates into H+ and OH– • Chemicals (acid/base) may shift amounts – Shift measured by p. H scale • p. H of solution affects biological functions © 2013 Pearson Education, Inc.

Increasingly basic (lower H+ concentration) 14 1. 0 M Na. OH Increasingly acidic (greater H+ concentration) Neutral [H+] = [OH–] 13 12 Ammonia Household ammonia 11 Milk of magnesia 10 Baking soda 9 8 Seawater Human blood 7 Pure water Milk 6 5 4 3 2 1 Tomatoes Wine Vinegar, colas Lemon juice Stomach acid 0 p. H scale © 2013 Pearson Education, Inc.

3. 2 The Organic Chemistry of Life • Organic molecules – Carbon atom covalently bonded to hydrogen and other atoms – Primary structural and function component of life – Range in size – Inorganic molecules not made of carbon and hydrogen © 2013 Pearson Education, Inc.

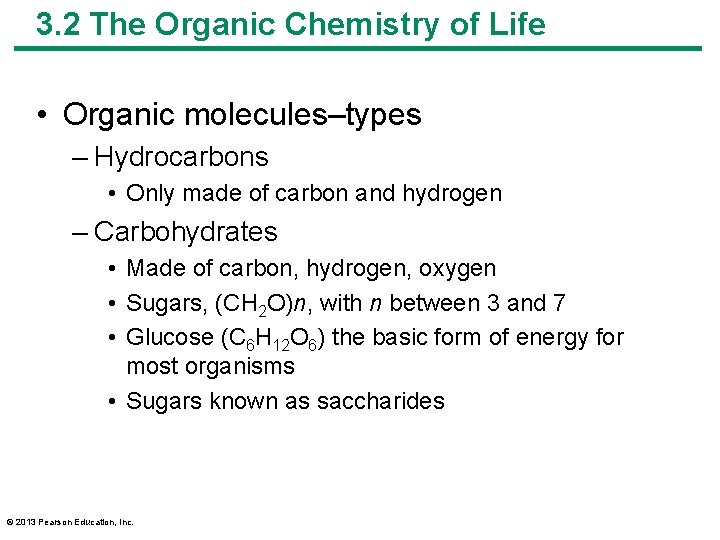
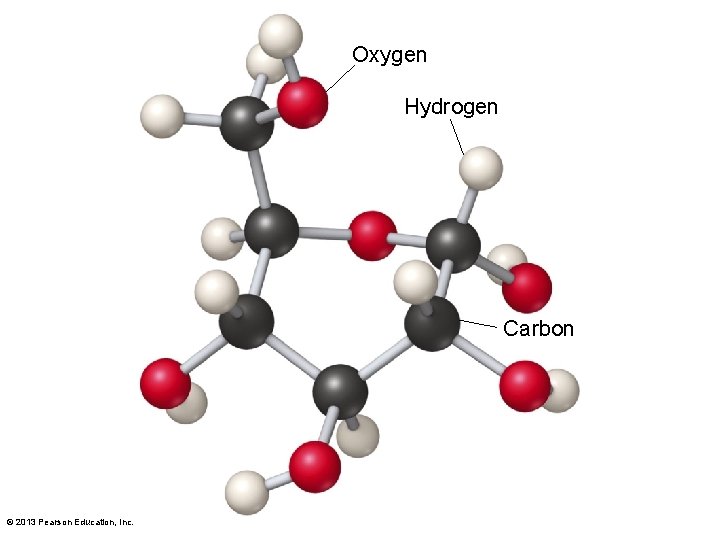
3. 2 The Organic Chemistry of Life • Organic molecules–types – Hydrocarbons • Only made of carbon and hydrogen – Carbohydrates • Made of carbon, hydrogen, oxygen • Sugars, (CH 2 O)n, with n between 3 and 7 • Glucose (C 6 H 12 O 6) the basic form of energy for most organisms • Sugars known as saccharides © 2013 Pearson Education, Inc.

Oxygen Hydrogen Carbon © 2013 Pearson Education, Inc.

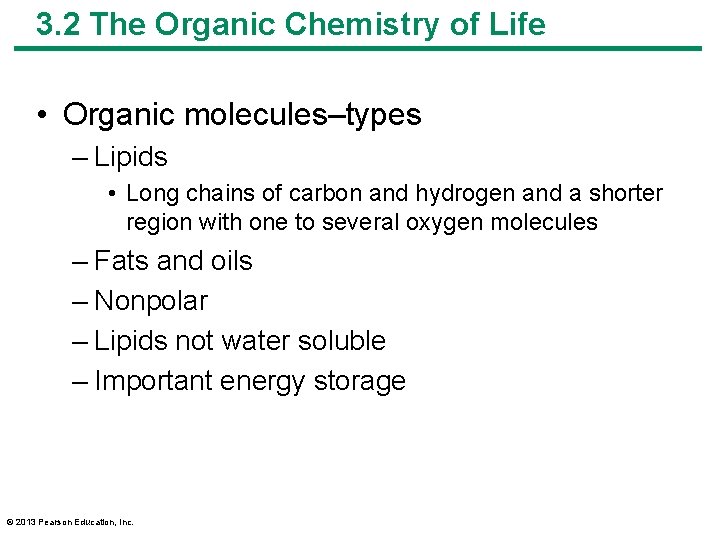
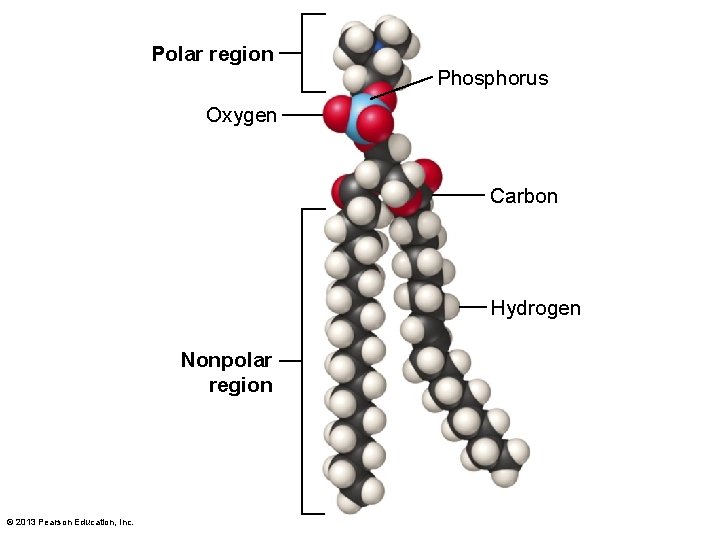
3. 2 The Organic Chemistry of Life • Organic molecules–types – Lipids • Long chains of carbon and hydrogen and a shorter region with one to several oxygen molecules – Fats and oils – Nonpolar – Lipids not water soluble – Important energy storage © 2013 Pearson Education, Inc.

Polar region Phosphorus Oxygen Carbon Hydrogen Nonpolar region © 2013 Pearson Education, Inc.

3. 2 The Organic Chemistry of Life • Macromolecules – Small organic molecule linked together • Polymers – Linked together in long chains • Polysaccharides – Polymers of simple sugars • Starch • Cellulose © 2013 Pearson Education, Inc.

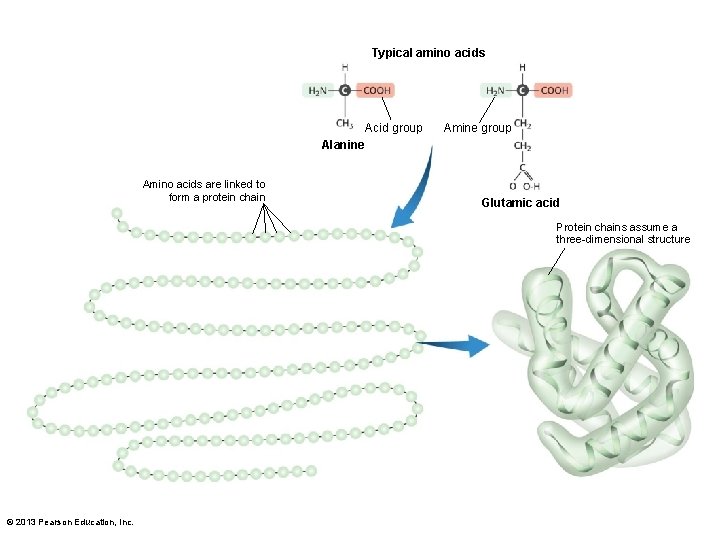
3. 2 The Organic Chemistry of Life • Macromolecules – Proteins • Polymers of amino acids • 20 amino acids–same base structure – Amino group (–NH 2) – Carboxylic acid group (–COOH) • Proteins made of chains of 100 to 1, 000+ amino acids © 2013 Pearson Education, Inc.

3. 2 The Organic Chemistry of Life • Macromolecules – Proteins – Fold to particular shapes yielding function – Structural or functional – May act as catalysts • Termed enzymes • Almost all biological reactions use enzymes © 2013 Pearson Education, Inc.

Typical amino acids Acid group Amine group Alanine Amino acids are linked to form a protein chain Glutamic acid Protein chains assume a three-dimensional structure © 2013 Pearson Education, Inc.

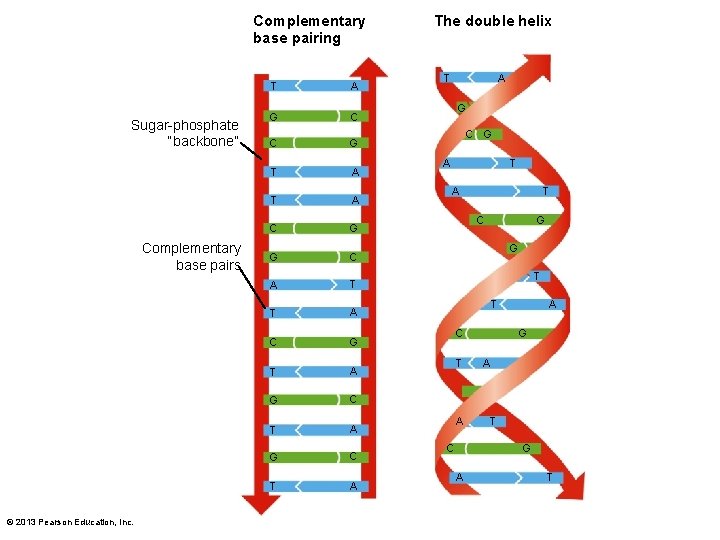
3. 2 The Organic Chemistry of Life • Nucleic acids – Polymers of nucleotides • 5 -carbon sugar, phosphate group, and nitrogenous base – Deoxyribonucleic acid (DNA) • Hereditary material • Traits coded in sequence of bases – adenine (A), thymine (T), cytosine (C), and guanine (G) © 2013 Pearson Education, Inc.

Complementary base pairing Sugar-phosphate “backbone” Complementary base pairs © 2013 Pearson Education, Inc. T A G C C G T A C G G C A T T A C G T A G C T A The double helix T A G C G A T C G G T T C T A C A G A T

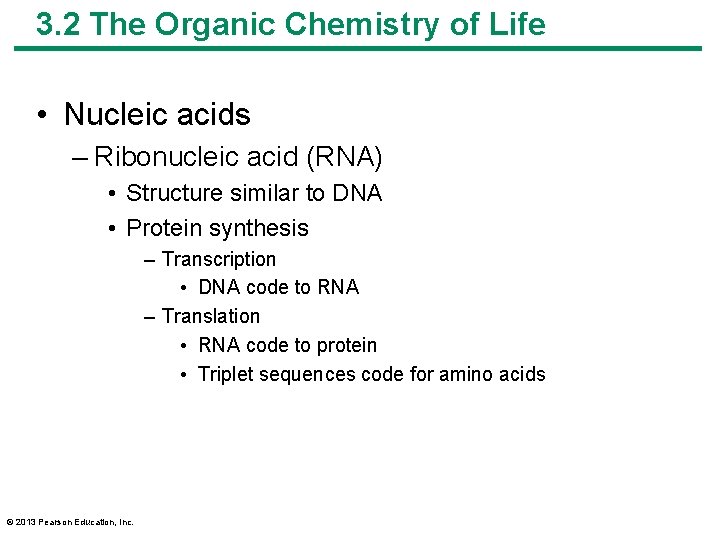
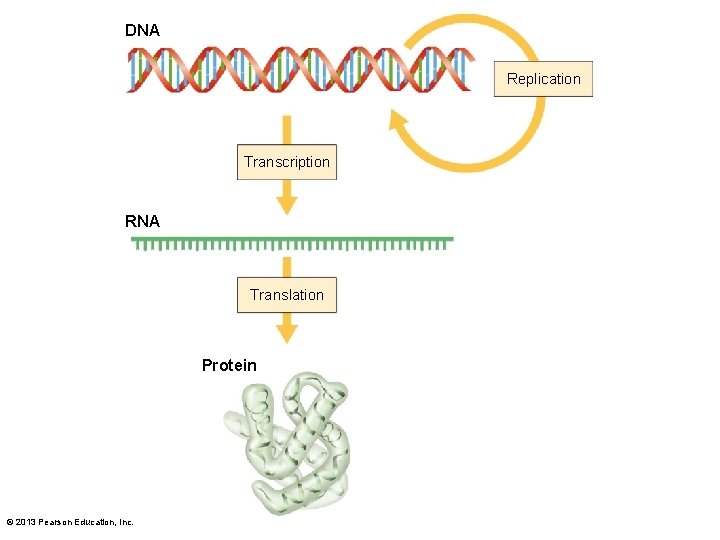
3. 2 The Organic Chemistry of Life • Nucleic acids – Ribonucleic acid (RNA) • Structure similar to DNA • Protein synthesis – Transcription • DNA code to RNA – Translation • RNA code to protein • Triplet sequences code for amino acids © 2013 Pearson Education, Inc.

DNA Replication Transcription RNA Translation Protein © 2013 Pearson Education, Inc.

3. 3 Energy and the Environment • Energy – Capacity to do work • Work – Force applied to an object over a distance – Potential energy • Stored energy – Kinetic energy • Energy in motion © 2013 Pearson Education, Inc.

3. 3 Energy and the Environment • Laws of Thermodynamics – First law • Energy can be neither created nor destroyed; can be transformed – Second law • Energy transformations increase disorder – Entropy – Energy often lost as heat © 2013 Pearson Education, Inc.

3. 3 Energy and the Environment • Consequences of the Laws of Thermodynamics – Total energy contained in the universe remains the same (energy conservation) – But, energy transformations result in increased disorder and useful energy is lost © 2013 Pearson Education, Inc.

3. 3 Energy and the Environment • Forms of energy • Four types important for ecosystems – Electromagnetic radiation • Energy moves as photons in waves • Electromagnetic spectrum–entire range of wavelengths – Gamma rays, X-rays, visible light, radiation, infrared, microwaves, radiowaves © 2013 Pearson Education, Inc.

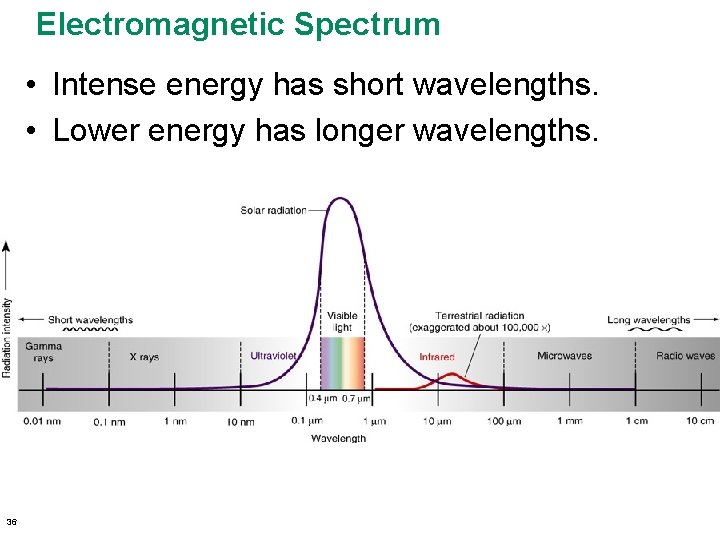
Electromagnetic Spectrum • Intense energy has short wavelengths. • Lower energy has longer wavelengths. 36

3. 3 Energy and the Environment • Forms of energy • Four types important for ecosystems – Heat • Kinetic energy of molecules • Temperature – Average kinetic energy © 2013 Pearson Education, Inc.

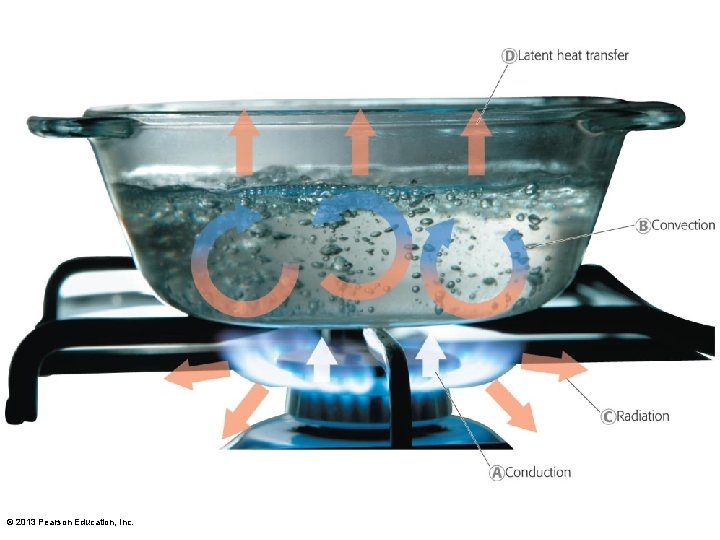
3. 3 Energy and the Environment • Forms of energy • Four types important for ecosystems – Heat can move in four ways • • Conduction Convection Radiation Latent heat transfer © 2013 Pearson Education, Inc.

© 2013 Pearson Education, Inc.

3. 3 Energy and the Environment • Forms of energy – Chemical energy • Potential energy • Breaking and forming of chemical bonds – Photosynthesis assembles carbohydrates – Potential energy in glucose bonds – When needed, energy released by respiration © 2013 Pearson Education, Inc.

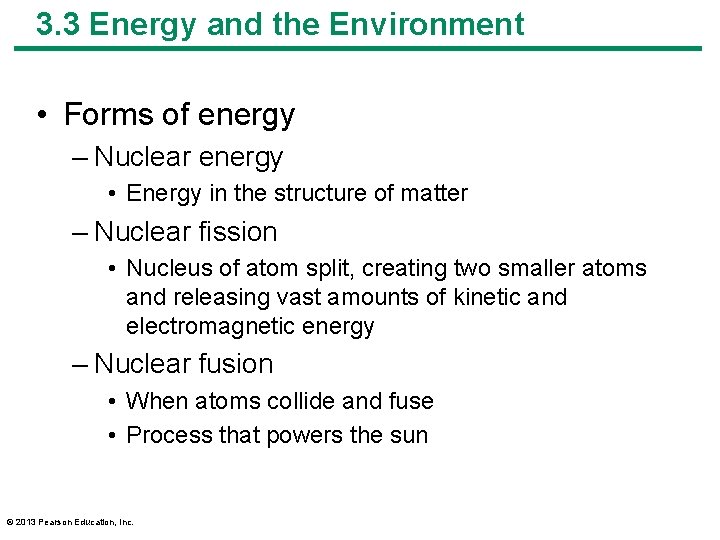
3. 3 Energy and the Environment • Forms of energy – Nuclear energy • Energy in the structure of matter – Nuclear fission • Nucleus of atom split, creating two smaller atoms and releasing vast amounts of kinetic and electromagnetic energy – Nuclear fusion • When atoms collide and fuse • Process that powers the sun © 2013 Pearson Education, Inc.

© 2013 Pearson Education, Inc.

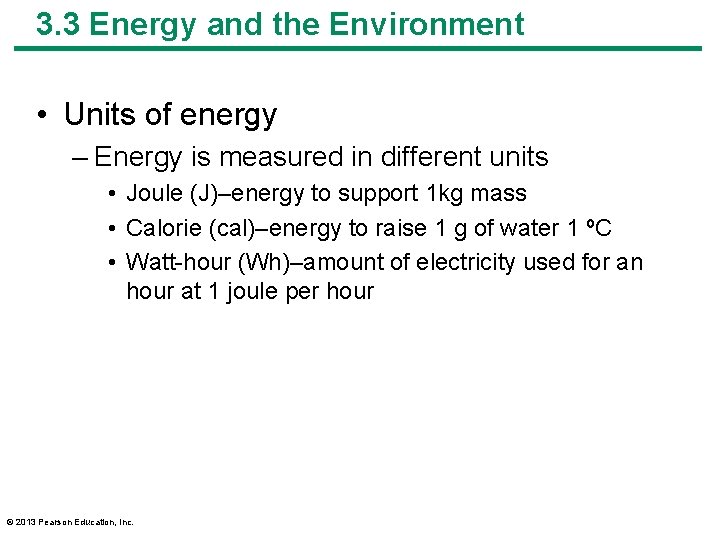
3. 3 Energy and the Environment • Units of energy – Energy is measured in different units • Joule (J)–energy to support 1 kg mass • Calorie (cal)–energy to raise 1 g of water 1 ºC • Watt-hour (Wh)–amount of electricity used for an hour at 1 joule per hour © 2013 Pearson Education, Inc.

3. 4 The Planet Earth in Context • Formation of solar system • Sun began forming 4. 6 billion years ago – The gases and dust not consumed by sun became planets and other objects • Planet-like objects • Asteroids • Comets © 2013 Pearson Education, Inc.

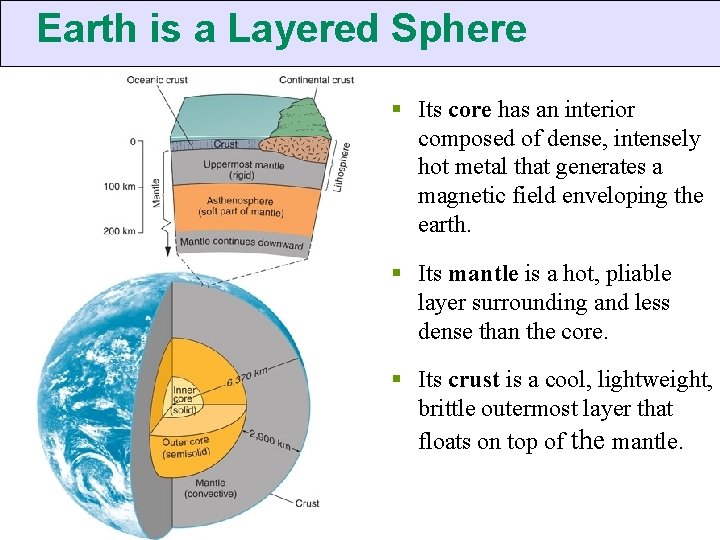
Earth is a Layered Sphere § Its core has an interior composed of dense, intensely hot metal that generates a magnetic field enveloping the earth. § Its mantle is a hot, pliable layer surrounding and less dense than the core. § Its crust is a cool, lightweight, brittle outermost layer that floats on top of the mantle.

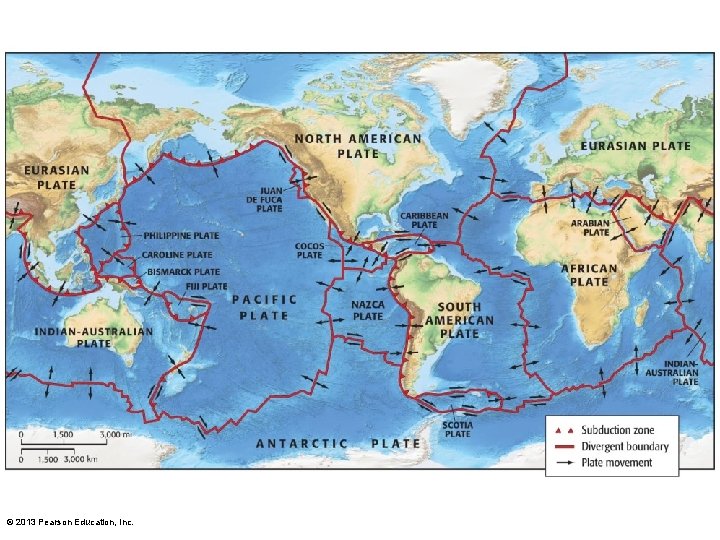
3. 5 Earth's Structure • Building and moving continents – Crust is slowly moving – Tectonic plates • Pieces of crust that float on mantle • Contents embedded in plates © 2013 Pearson Education, Inc.

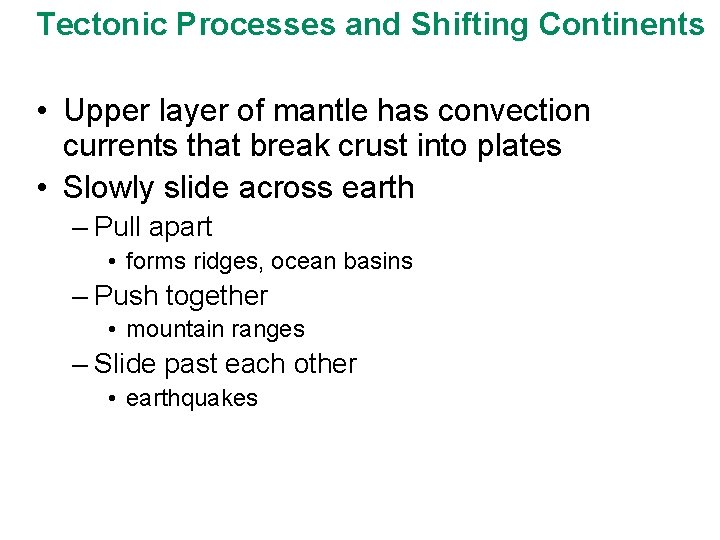
Tectonic Processes and Shifting Continents • Upper layer of mantle has convection currents that break crust into plates • Slowly slide across earth – Pull apart • forms ridges, ocean basins – Push together • mountain ranges – Slide past each other • earthquakes

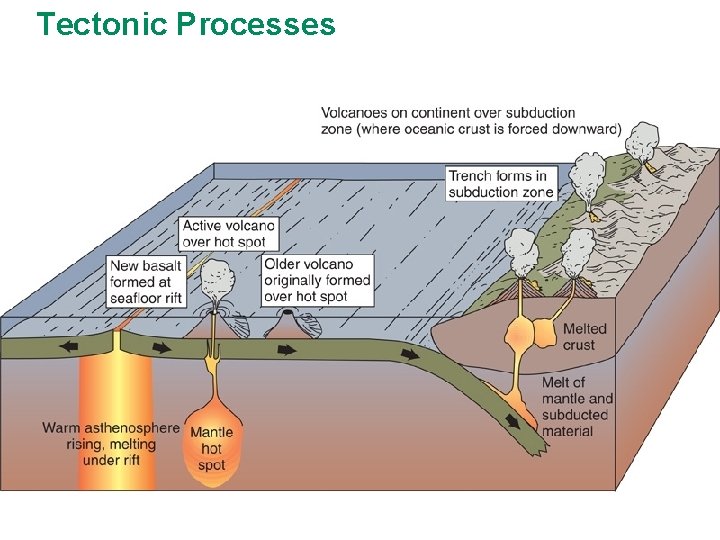
• Oceanic plate collides with Continental – continental rides up over seafloor – oceanic plate subducts and melts, rise back as magma • Subduction zones - deep ocean trenches and volcanoes

Tectonic Processes

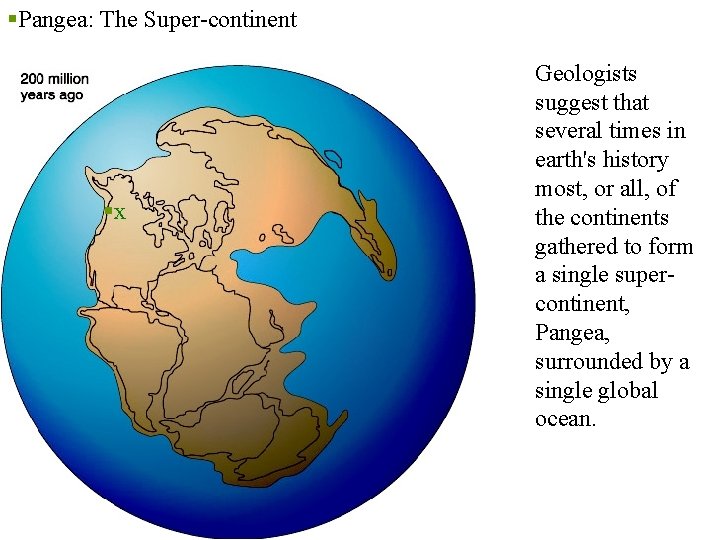
§Pangea: The Super-continent §x Geologists suggest that several times in earth's history most, or all, of the continents gathered to form a single supercontinent, Pangea, surrounded by a single global ocean.

© 2013 Pearson Education, Inc.

3. 5 Earth's Structure – Tectonic plates meet at boundaries • Transform fault boundaries – Plates slide past – Sites of earthquakes – Divergent boundaries • Plates spread apart – Convergent boundaries • Plates come together © 2013 Pearson Education, Inc.

3. 6 Earth's Atmosphere • Earth has gravity to hold atmosphere • Earth's unique atmosphere supports life – Composition of gases • • 78% nitrogen 21 % oxygen 0. 039% carbon dioxide Water vapor © 2013 Pearson Education, Inc.

3. 6 Earth's Atmosphere • Layers of atmosphere – 480 km deep (300 miles) – Air at surface compressed by gases above – Atmospheric pressure – Pressure decreases as altitude increases © 2013 Pearson Education, Inc.

3. 6 Earth's Atmosphere • Layers of atmosphere – Troposphere • Lowest layer • Life located here • Temperature drops with elevation – Stratosphere • 11– 48 km • Temperature increases approaching ozone • Ozone layer located here – Protects life from ultraviolet radiation © 2013 Pearson Education, Inc.

3. 6 Earth's Atmosphere • Layers of atmosphere – Mesosphere • Above stratosphere • Air temperature drops again (173 ºC) at 90 km – Thermosphere • • Extends out to space Above 150 km gas density so low no friction International Space Station orbits here Aurora occurs here © 2013 Pearson Education, Inc.

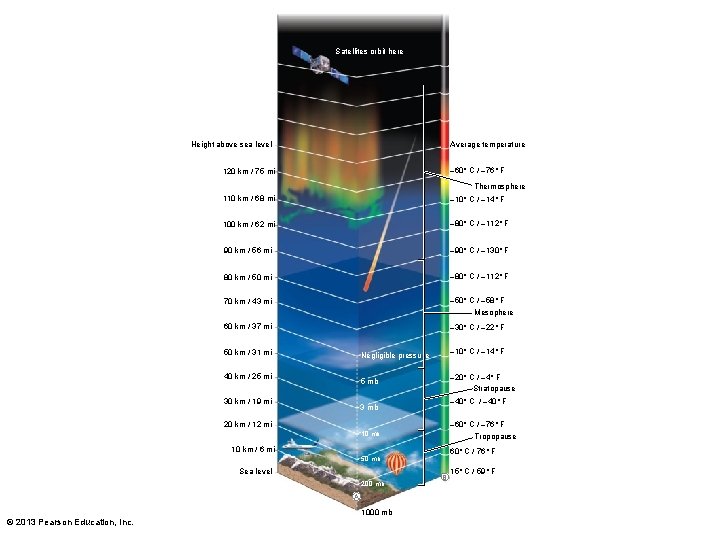
Satellites orbit here Height above sea level Average temperature 120 km / 75 mi – 60° C / – 76° F 110 km / 68 mi – 10° C / – 14° F 100 km / 62 mi – 80° C / – 112° F 90 km / 56 mi – 90° C / – 130° F 80 km / 50 mi – 80° C / – 112° F 70 km / 43 mi – 50° C / – 58° F Thermosphere Mesophere 60 km / 37 mi – 30° C / – 22° F 50 km / 31 mi – 10° C / – 14° F Negligible pressure 40 km / 25 mi – 20° C / – 4° F Stratopause 5 mb 30 km / 19 mi – 40° C / – 40° F 3 mb 20 km / 12 mi – 60° C / – 76° F 10 mb Tropopause 10 km / 6 mi 60° C / 76° F 50 mb Sea level 200 mb A © 2013 Pearson Education, Inc. 1000 mb B 15° C / 59° F

The atmosphere has four distinct zones of contrasting temperature.

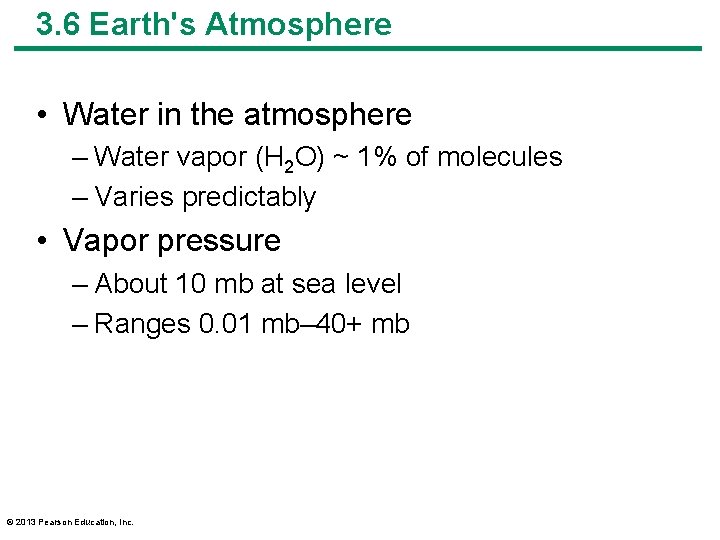
3. 6 Earth's Atmosphere • Water in the atmosphere – Water vapor (H 2 O) ~ 1% of molecules – Varies predictably • Vapor pressure – About 10 mb at sea level – Ranges 0. 01 mb– 40+ mb © 2013 Pearson Education, Inc.

3. 6 Earth's Atmosphere • Saturation vapor pressure – Temperature dependent • Amount of water air can hold raises with temperature – Above saturation vapor pressure – Water condenses • Rain, fog © 2013 Pearson Education, Inc.

© 2013 Pearson Education, Inc.

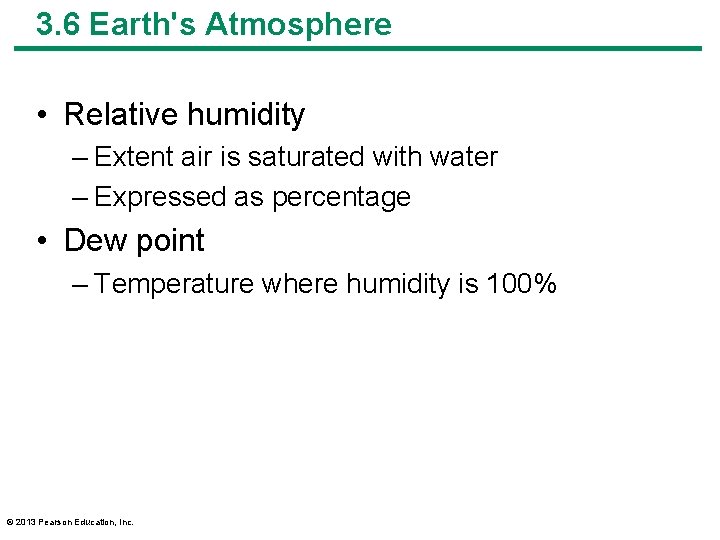
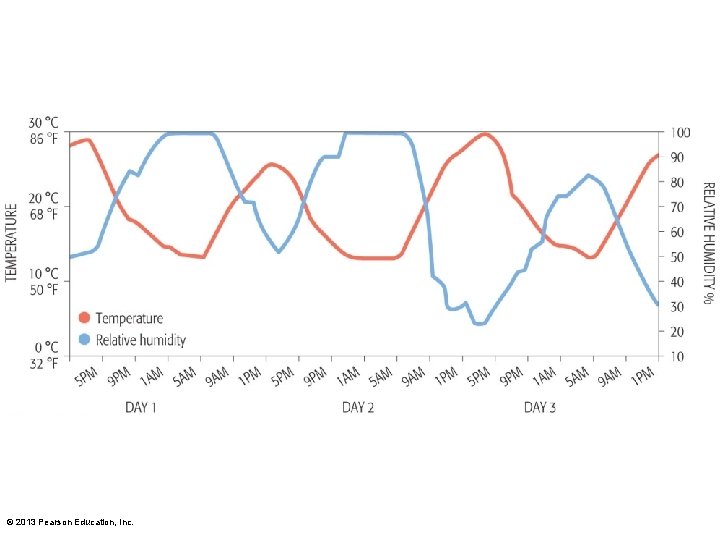
3. 6 Earth's Atmosphere • Relative humidity – Extent air is saturated with water – Expressed as percentage • Dew point – Temperature where humidity is 100% © 2013 Pearson Education, Inc.

© 2013 Pearson Education, Inc.

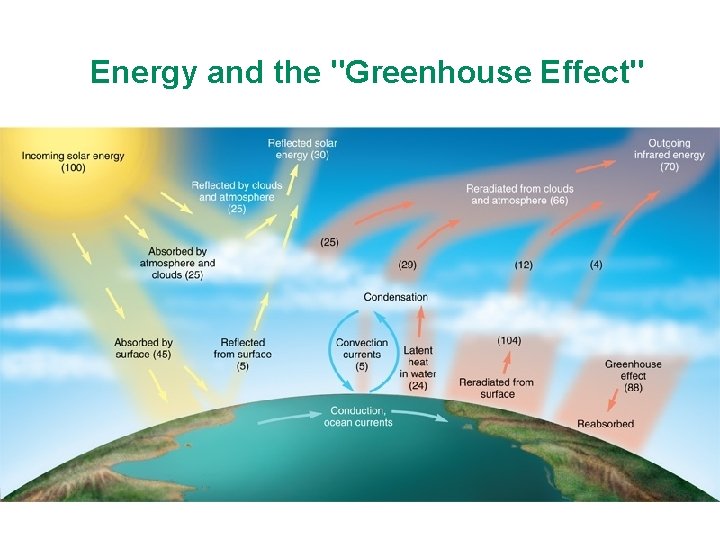
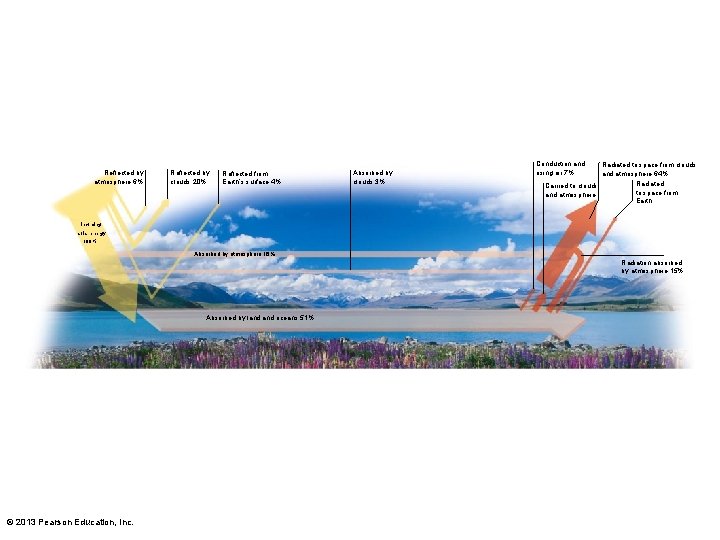
3. 7 Earth's Energy Budget, Weather, and Climate • Energy budget – Measures all energy entering and leaving Earth – Balances over time • Solar radiation – 30% reflected to space – 70% absorbed by land, sea, and air • Absorbed heat eventually radiated back to space as infrared radiation © 2013 Pearson Education, Inc.

Energy and the "Greenhouse Effect"

Reflected by atmosphere 6% Reflected by clouds 20% Reflected from Earth’s surface 4% Absorbed by clouds 3% Conduction and rising air 7% Radiated to space from clouds and atmosphere 64% Radiated Carried to clouds to space from and atmosphere Earth Incoming solar energy 100% Absorbed by atmosphere 16% Radiation absorbed by atmosphere 15% Absorbed by land oceans 51% © 2013 Pearson Education, Inc.

3. 7 Earth's Energy Budget, Weather, and Climate • Weather and climate – Climate • Long-term atmospheric conditions – Temperature, humidity, average rainfall – Weather • Short-term variations local atmospheric conditions – Thunderstorms © 2013 Pearson Education, Inc.

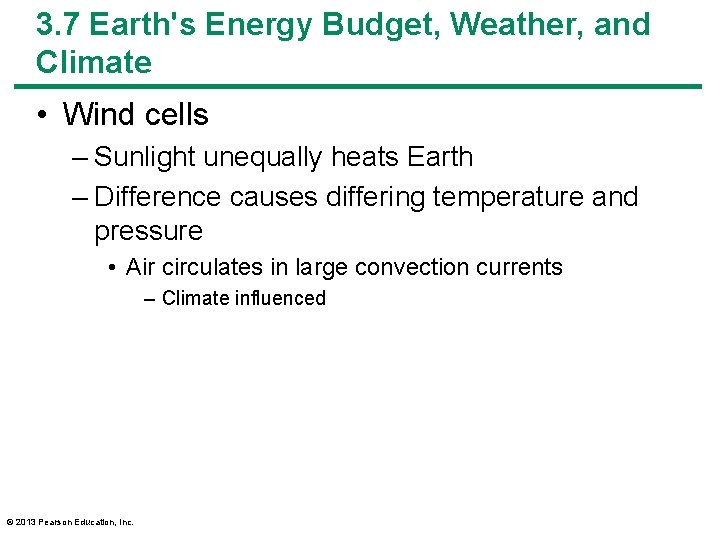
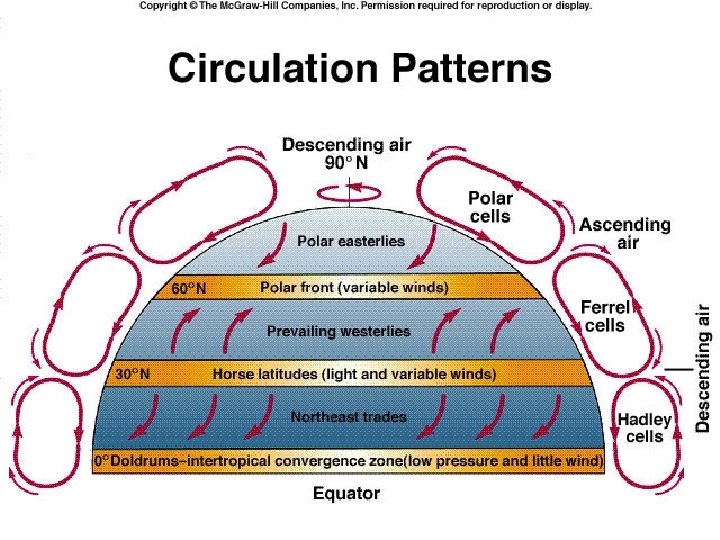
3. 7 Earth's Energy Budget, Weather, and Climate • Wind cells – Sunlight unequally heats Earth – Difference causes differing temperature and pressure • Air circulates in large convection currents – Climate influenced © 2013 Pearson Education, Inc.

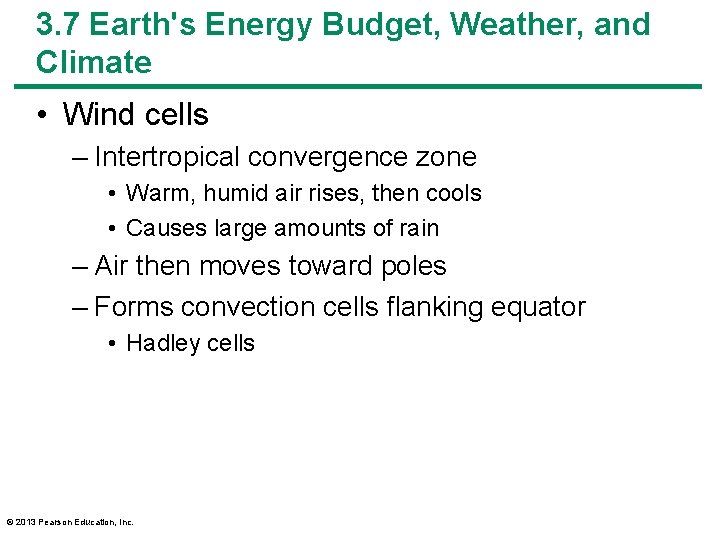
3. 7 Earth's Energy Budget, Weather, and Climate • Wind cells – Intertropical convergence zone • Warm, humid air rises, then cools • Causes large amounts of rain – Air then moves toward poles – Forms convection cells flanking equator • Hadley cells © 2013 Pearson Education, Inc.

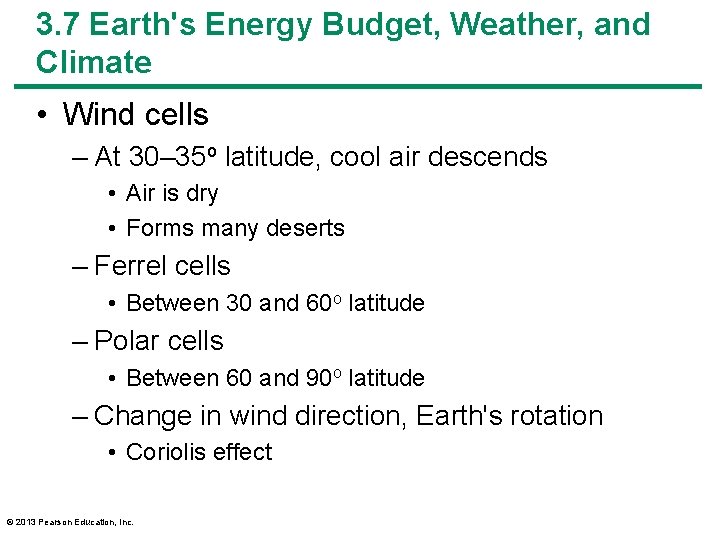
3. 7 Earth's Energy Budget, Weather, and Climate • Wind cells – At 30– 35 o latitude, cool air descends • Air is dry • Forms many deserts – Ferrel cells • Between 30 and 60 o latitude – Polar cells • Between 60 and 90 o latitude – Change in wind direction, Earth's rotation • Coriolis effect © 2013 Pearson Education, Inc.

Convection and Atmospheric Pressure • • Evaporation Latent heat Condensation Convection currents • Air pressure differences • Coriolis effect



Tornadoes are local cyclonic storms caused by rapid mixing of cold, dry air and warm, wet air.


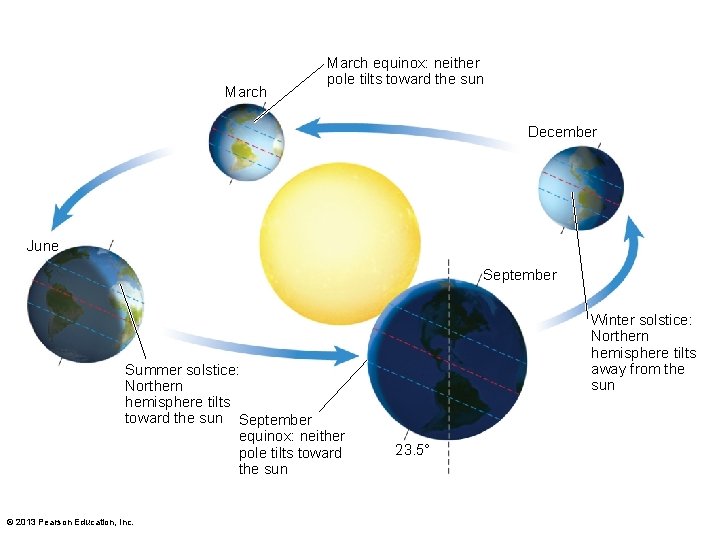
3. 7 Earth's Energy Budget, Weather, and Climate • Earth tilted on axis 23. 5 degrees – Causes differential heating throughout the year – Causes wind cells to shift north or south • Differences in rainfall and temperature • Temperature difference more extreme at center of continents and higher at latitudes © 2013 Pearson Education, Inc.

March equinox: neither pole tilts toward the sun December June September Summer solstice: Northern hemisphere tilts toward the sun September equinox: neither pole tilts toward the sun © 2013 Pearson Education, Inc. Winter solstice: Northern hemisphere tilts away from the sun 23. 5°