Chapter 3 The Driving Forces Exothermic Endothermic Entropy















- Slides: 27

Chapter 3 The Driving Forces Exothermic & Endothermic Entropy

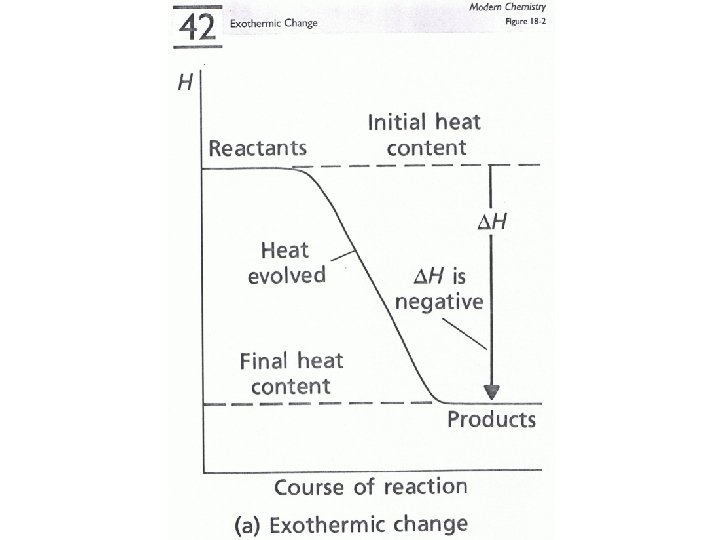
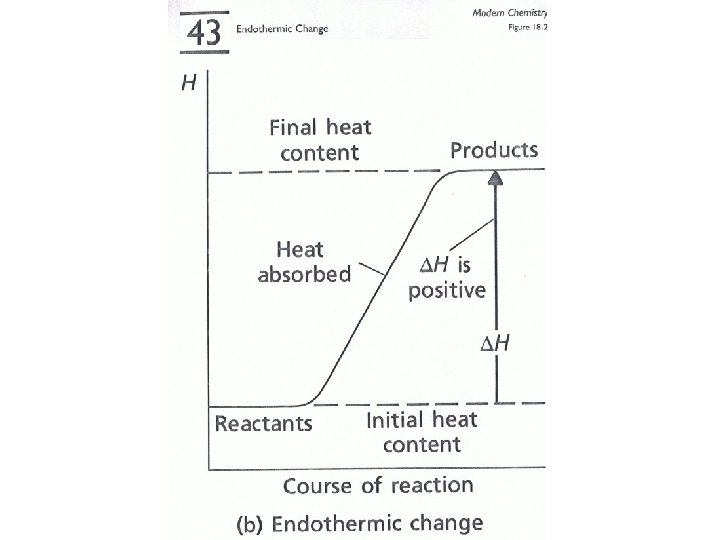
• All changes whether physical or chemical involve a change in energy. • Energy can be released during the change (exothermic). • Energy may be absorbed during the change (endothermic). • The amount of energy that is released or absorbed in measured in joules or calories.



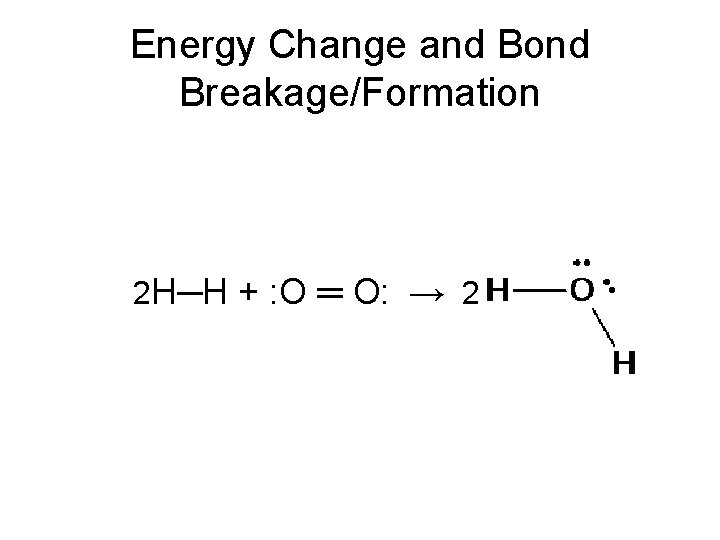
Energy Change and Bond Breakage/Formation

Energy Change and Bond Breakage/Formation • Bonds are energy. • Each different type of bond has an amount of energy associated with it.

Energy Change and Bond Breakage/Formation • Bonds are energy. • Each different type of bond has an amount of energy associated with it. • When we break a bond the process requires energy (it is endothermic). • When we form a bond the process releases energy (it is exothermic). • The net energy change determines whether the reaction is endothermic or exothermic.

Energy Change and Bond Breakage/Formation 2 H─H + : O ═ O: → 2

Energy Change and Bond Breakage/Formation • We can often determine whether energy is being released or absorbed by a reaction by determining if any temperature change occurs.

Temperature Change • Exothermic reactions • Endothermic reactions “often” feel hot. “often” feel cold.

See the page in your notes titled “The Driving Forces”

Spontaneous vs. Nonspontaneous • A spontaneous process is a process that occurs without outside intervention. • No external forces are required to keep the process going, although external forces may be required to get the process started.

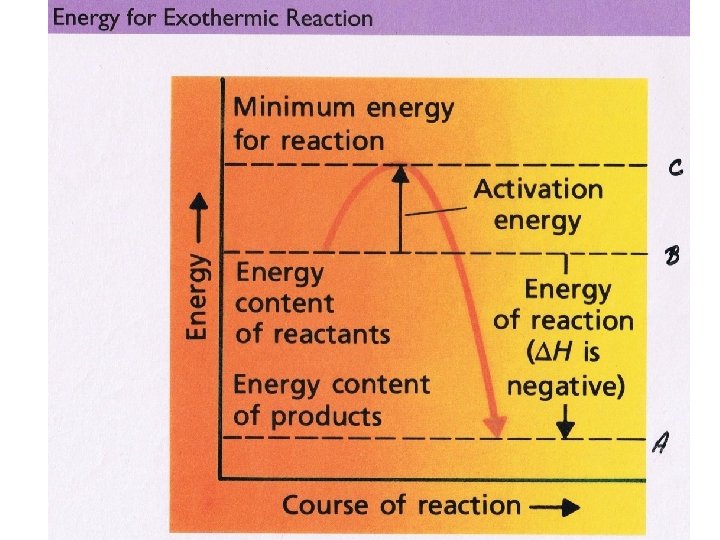
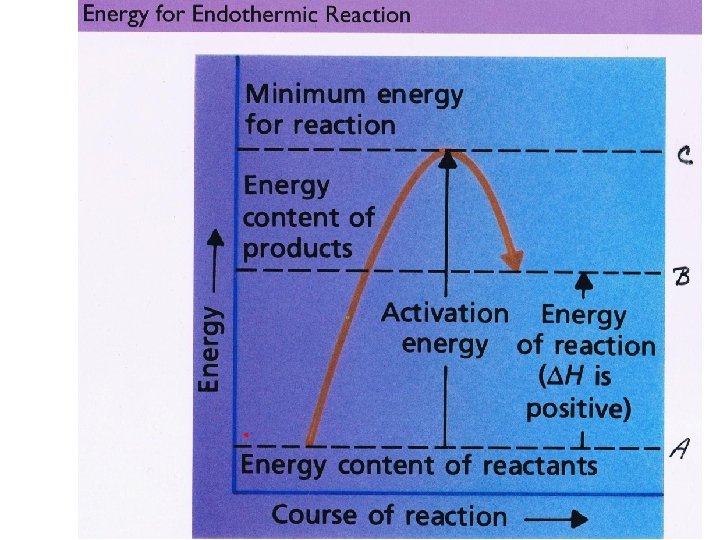
Activation Energy • The activation energy is the amount of energy that must be added to a reaction for it to occur. • Both exothermic and endothermic reactions require activation energy.



Spontaneous vs. Non-spontaneous • A non-spontaneous process will not occur naturally.

The Driving Forces • All reactions (changes) in nature occur because of the interplay of two driving forces: (1) The drive toward lower energy. (2) The drive toward increased randomness.

Entropy • Entropy is the amount of randomness (lack of order) in a system. Some call it entropy. I call it heaven

Entropy • Entropy is how “chaotic”, (disordered), a system is. Some call it entropy. I call it heaven

Increase in Entropy

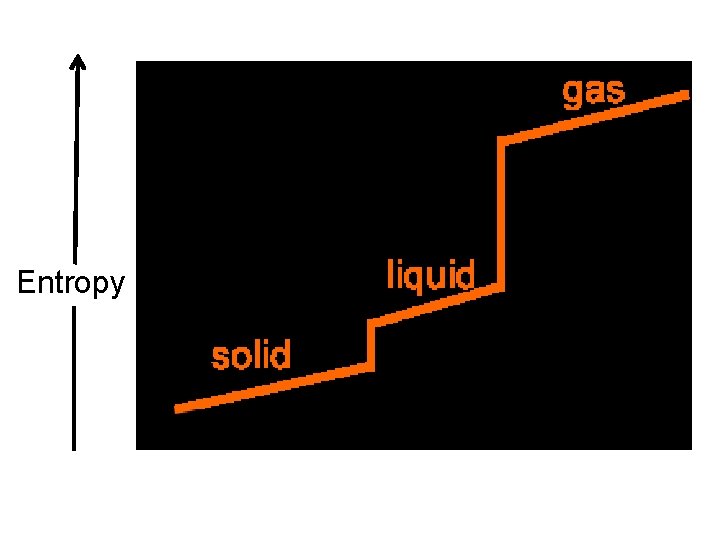
Increase in Entropy • Production of liquid or gas from a solid.

Increase in Entropy • Production of gas from a liquid.

Entropy

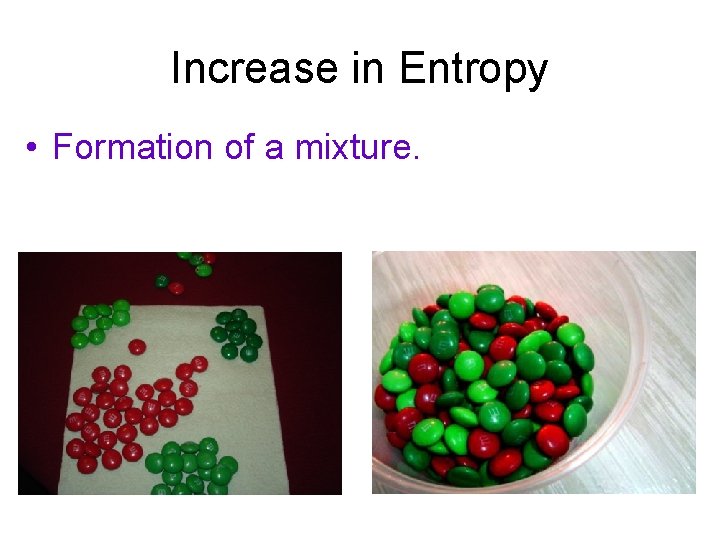
Increase in Entropy • Formation of a mixture.

Increase in Entropy • More particles are created.

The mixing of the white solids which I did earlier was spontaneous. Why?

Release Energy Increase Entropy