Chapter 3 The Composition of Molecules Department of













- Slides: 28

Chapter 3 The Composition of Molecules Department of Chemistry and Biochemistry Seton Hall University

Molecules • The sizes and shapes of molecules are a key determinant of their physical and chemical properties • A variety of chemical properties can be displayed by compounds consisting of only a few elements • Even simple molecules can have vastly different properties (consider O 2, CO and CO 2 2

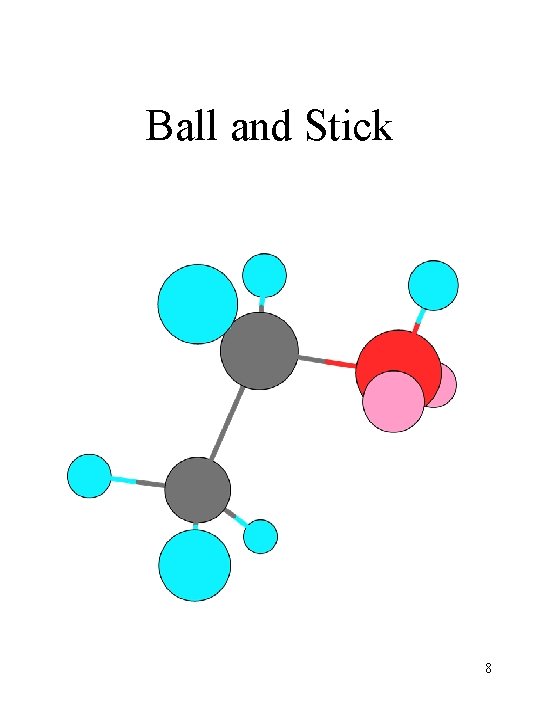
Representing molecules • Ways of indicating structure – structural formulas – ball and stick structures – space-filling structures – line structures • Each has its uses; you will need to learn all of them 3

Chemical formulas • indicate relative numbers of atoms of each element • for substances that form discrete molecules, the chemical formula is also the molecular formula • contains elemental symbols to represent the elements • subscripts indicate the number of each element 4

Chemical formulas • Since chemical compounds contain two or more different elements, the order of the elements in the formula is an issue • Element that occurs furthest to the left of the periodic table is normally listed first 5

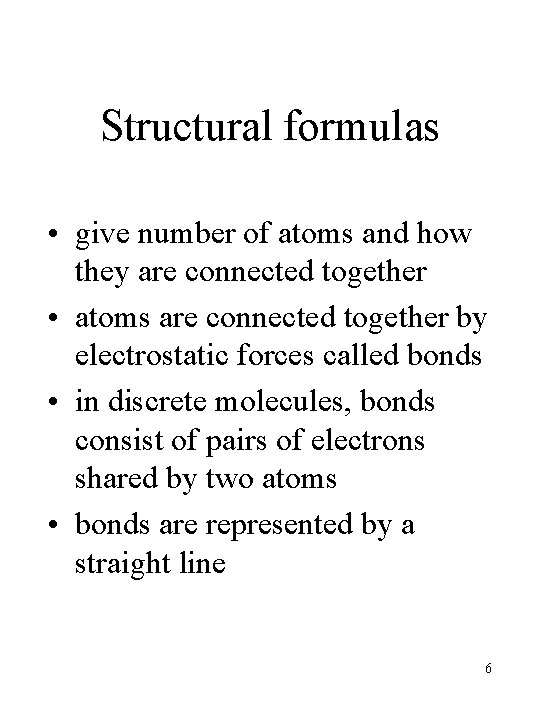
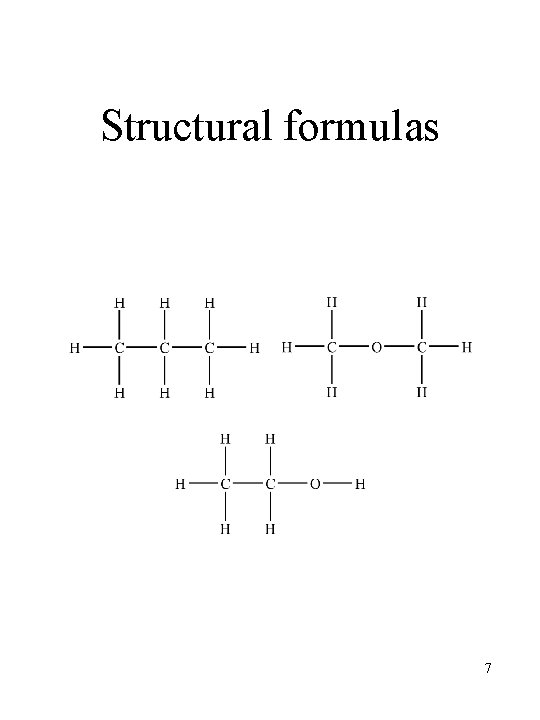
Structural formulas • give number of atoms and how they are connected together • atoms are connected together by electrostatic forces called bonds • in discrete molecules, bonds consist of pairs of electrons shared by two atoms • bonds are represented by a straight line 6

Structural formulas 7

Ball and Stick 8

Space-filling model 9

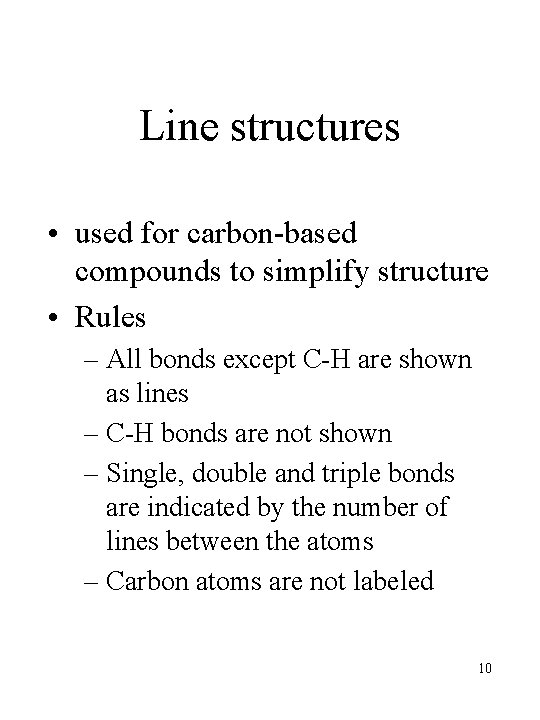
Line structures • used for carbon-based compounds to simplify structure • Rules – All bonds except C-H are shown as lines – C-H bonds are not shown – Single, double and triple bonds are indicated by the number of lines between the atoms – Carbon atoms are not labeled 10

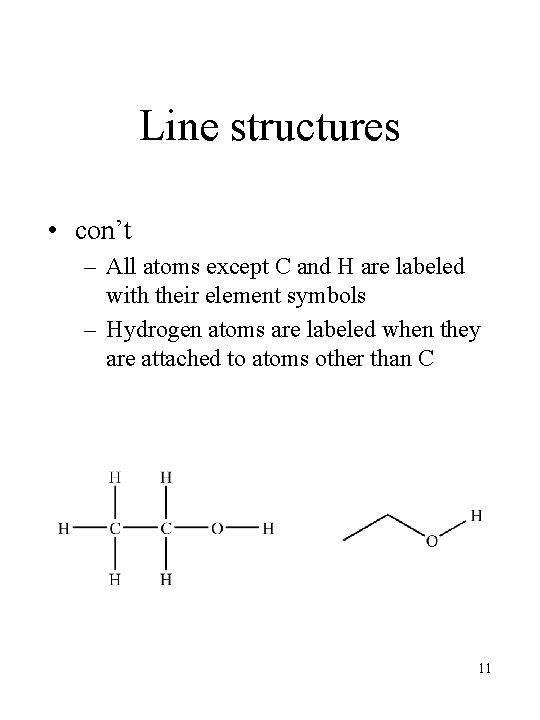
Line structures • con’t – All atoms except C and H are labeled with their element symbols – Hydrogen atoms are labeled when they are attached to atoms other than C 11

Physical states of elements • Reference condition is 1 atmosphere of pressure and 25 °C • Gaseous elements - H 2, N 2, O 2, F 2, Cl 2, noble gases • Liquid elements - Hg, Br 2 • All other elements are solids • Some elements occur as atomic clusters (S 8, P 4) 12

Binary compound nomenclature • Binary compounds contain two different elements • Three types of binary compounds – Metals exhibiting only one oxidation state forming a compound with a nonmetal – Metals exhibiting two or more oxidation states forming a compound with a nonmetal – Compounds of nonmetals and nonmetals 13

Metals with only one oxidation state • Groups of metals with only one common oxidation state – alkali metals - +1 – alkaline earths - +2 – Ag - +1 – Cd, Zn - +2 – Al - +3 • All other metals can exhibit more that one oxidation state 14

Anions in negative oxidation states • Nonmetallic anions usually exhibit one negative oxidation state – halogens – chalcogens – pnicogens – carbides -1 -2 -3 -4 15

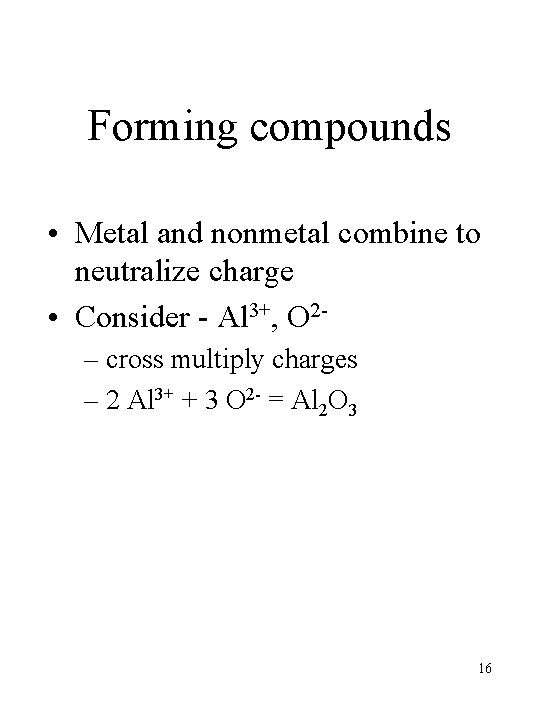
Forming compounds • Metal and nonmetal combine to neutralize charge • Consider - Al 3+, O 2– cross multiply charges – 2 Al 3+ + 3 O 2 - = Al 2 O 3 16

Naming compounds • Use name of metal with no changes • Change the name of the anion by taking the “stem” and add the suffix -ide • Examples – Na. Cl - sodium chloride – Mg. Cl 2 - magnesium chloride 17

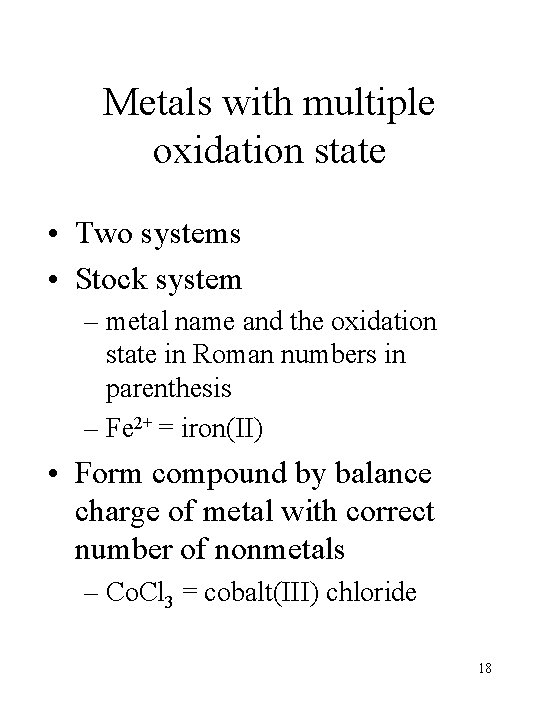
Metals with multiple oxidation state • Two systems • Stock system – metal name and the oxidation state in Roman numbers in parenthesis – Fe 2+ = iron(II) • Form compound by balance charge of metal with correct number of nonmetals – Co. Cl 3 = cobalt(III) chloride 18

Classical nomenclature • Metals in multiple oxidation states usually have one or two common oxidation states • First row metals are +2 and +3 (except Cu 2+ and Cu+) • use -ous suffix for lower common oxidation state • use -ic suffix for higher common oxidation state 19

Examples • Co. Cl 3 - cobaltic chloride • Ni. Cl 2 - nickelous chloride • For metals with Latin names, use them • Cu. Cl - cuprous chloride • Fe. Br 3 - ferric bromide 20

Nonmetals + nonmetals • Name nonmetal further to the left of the periodic table first with no changes • Name nonmetal further to the right of the periodic table second with the -ide suffix • Use Greek prefixes to indicate the number of each one 21

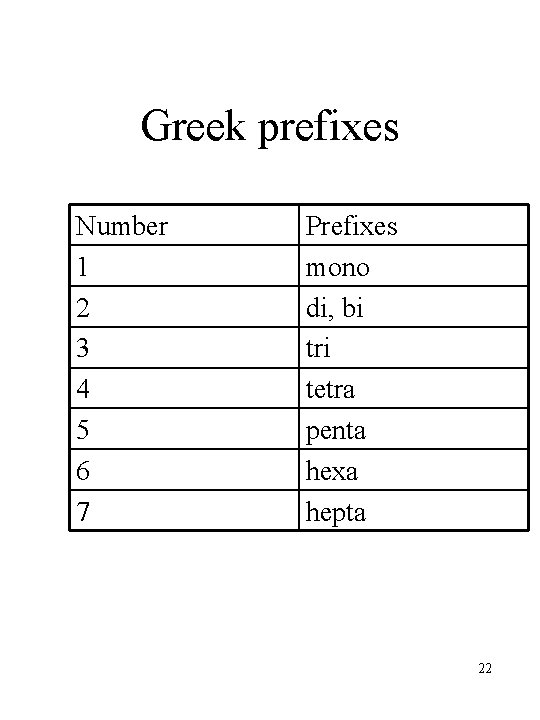
Greek prefixes Number 1 2 3 4 5 6 7 Prefixes mono di, bi tri tetra penta hexa hepta 22

Examples • N 2 O 3 - dinitrogen trioxide • CO 2 - carbon dioxide • P 2 O 5 - diphosphorus pentoxide 23

Oxy anions • anions composed of oxygen and another elements • other elements can be a metal or a nonmetals • Examples – SO 42 -, NO 2 - • Note that oxyanions that have 2 or greater negative charge can bind a proton – HCO 3 - - hydrogen carbonate – HSO 4 - - hydrogen sulfate 24

Naming • Need common oxidation states – most common oxidation state for nonmetals is the group number (except for the halogens) – next most common oxidation state is the group number minus one • use -ate suffix for higher oxidation state and -ite suffix for next higher oxidation state 25

Examples • • • SO 42 - - sulfate SO 32 - - sulfite NO 3 - - nitrate NO 2 - - nitrite Salts with these oxyanions – Na 2 SO 4 - sodium sulfate – KNO 3 - potassium nitrate 26

Acids • Binary acids – name begins with hydro – then add stem of nonmetal plus -ic – end with acid • Examples – HCl - hydrochloric acid – H 2 S - hydrosulfuric acid 27

Oxyacids • Take oxyanion suffix and convert – change -ate to -ic – change -ite to -ous • Do not use hydro- in the beginning • Examples – H 2 SO 4 - sulfuric acid – H 2 SO 3 - sulfurous acid 28