Chapter 3 Stoichiometry Copyright 2018 Cengage Learning All



























































- Slides: 158

Chapter 3 Stoichiometry Copyright © 2018 Cengage Learning. All Rights Reserved.

Chapter 3 Table of Contents § § § (3. 1) (3. 2) (3. 3) (3. 4) (3. 5) (3. 6) Counting by weighing Atomic masses The mole Molar mass Learning to solve problems Percent composition of compounds Copyright © 2018 Cengage Learning. All Rights Reserved.

Chapter 3 Table of Contents § § (3. 7) (3. 8) (3. 9) (3. 10) § (3. 11) Determining the formula of a compound Chemical equations Balancing chemical equations Stoichiometric calculations: Amounts of reactants and products The concept of limiting reactant Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 1 Counting by Weighing Chemical Stoichiometry § Study of the quantities of materials consumed and produced in chemical reactions § Requires understanding the concept of relative atomic masses Copyright © Cengage Learning. All rights reserved Copyright © 2018 Cengage Learning. All Rights Reserved. 4

Section 3. 1 Counting by Weighing Counting Objects by Their Mass § Average mass of objects is required to count the objects by weighing § For purposes of counting, objects behave as though they are all identical § Samples of matter can contain huge numbers of atoms § Number of atoms in a sample can be determined by finding its mass Copyright © Cengage Learning. All rights reserved Copyright © 2018 Cengage Learning. All Rights Reserved. 5

Section 3. 2 Atomic Masses Modern System of Atomic Masses § Instituted in 1961 § Standard - 12 C § 12 C is assigned a mass of exactly 12 atomic mass units (u) § Masses of all other atoms are given relative to this standard Copyright © 2018 Cengage Learning. All Rights Reserved.

Chapter 3 Stoichiometry Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 2 Atomic Masses Mass Spectrometer (continued 1) § Atoms or molecules are passed into a beam of high-speed electrons § Electrons are knocked off the atoms or molecules being analyzed and are changed into positive ions § An applied electric field then accelerates these ions into a magnetic field Copyright © 2018 Cengage Learning. All Rights Reserved.

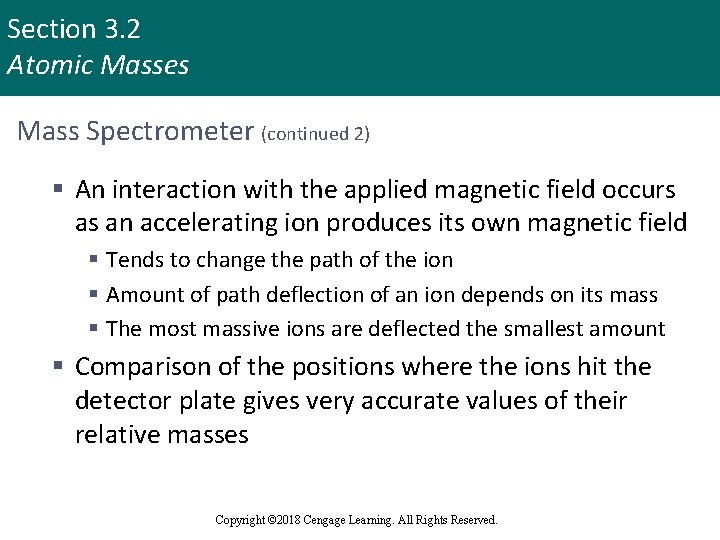
Section 3. 2 Atomic Masses Mass Spectrometer (continued 2) § An interaction with the applied magnetic field occurs as an accelerating ion produces its own magnetic field § Tends to change the path of the ion § Amount of path deflection of an ion depends on its mass § The most massive ions are deflected the smallest amount § Comparison of the positions where the ions hit the detector plate gives very accurate values of their relative masses Copyright © 2018 Cengage Learning. All Rights Reserved.

Chapter 3 Stoichiometry Copyright © 2018 Cengage Learning. All Rights Reserved.

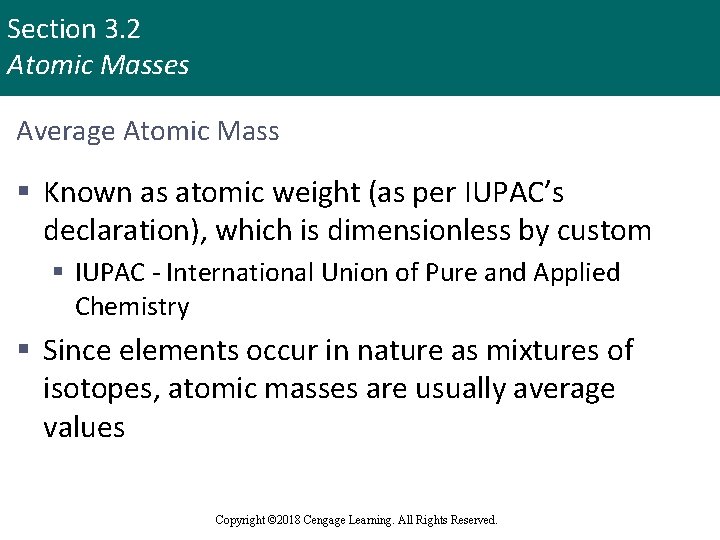
Section 3. 2 Atomic Masses Average Atomic Mass § Known as atomic weight (as per IUPAC’s declaration), which is dimensionless by custom § IUPAC - International Union of Pure and Applied Chemistry § Since elements occur in nature as mixtures of isotopes, atomic masses are usually average values Copyright © Cengage Learning. All rights reserved Copyright © 2018 Cengage Learning. All Rights Reserved. 11

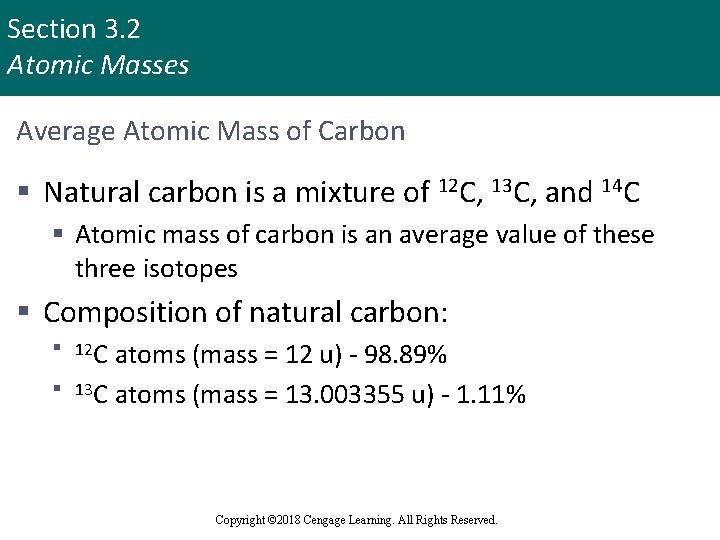
Section 3. 2 Atomic Masses Average Atomic Mass of Carbon § Natural carbon is a mixture of 12 C, 13 C, and 14 C § Atomic mass of carbon is an average value of these three isotopes § Composition of natural carbon: § 12 C atoms (mass = 12 u) - 98. 89% § 13 C atoms (mass = 13. 003355 u) - 1. 11% Copyright © Cengage Learning. All rights reserved Copyright © 2018 Cengage Learning. All Rights Reserved. 12

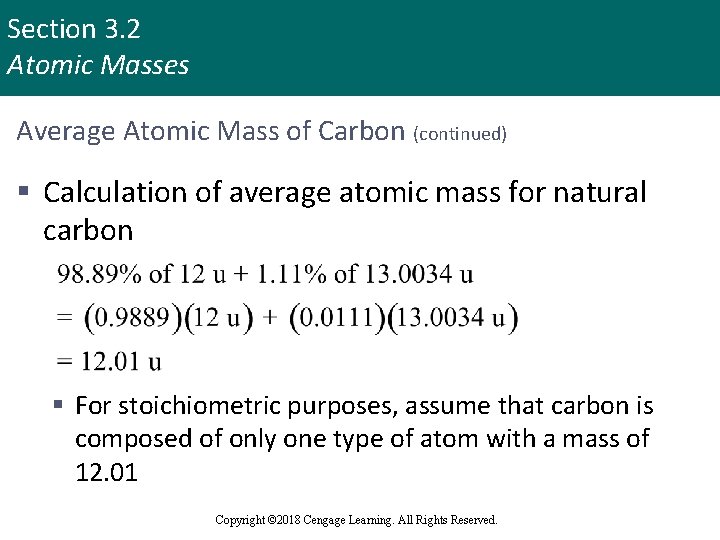
Section 3. 2 Atomic Masses Average Atomic Mass of Carbon (continued) § Calculation of average atomic mass for natural carbon § For stoichiometric purposes, assume that carbon is composed of only one type of atom with a mass of 12. 01 Copyright © Cengage Learning. All rights reserved Copyright © 2018 Cengage Learning. All Rights Reserved. 13

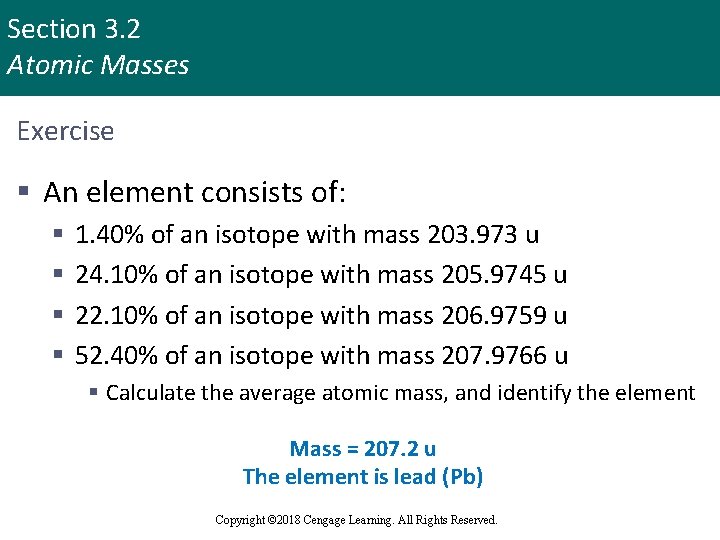
Section 3. 2 Atomic Masses Exercise § An element consists of: § § 1. 40% of an isotope with mass 203. 973 u 24. 10% of an isotope with mass 205. 9745 u 22. 10% of an isotope with mass 206. 9759 u 52. 40% of an isotope with mass 207. 9766 u § Calculate the average atomic mass, and identify the element Mass = 207. 2 u The element is lead (Pb) Copyright © Cengage Learning. All rights reserved Copyright © 2018 Cengage Learning. All Rights Reserved. 14

Chapter 3 Stoichiometry Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 2 Atomic Masses Example 3. 1 - Solution § Where are we going? § To calculate the average mass of natural copper § What do we know? § 63 Cu mass = 62. 93 u § 65 Cu mass = 64. 93 u § How do we get there? § As shown by the graph, of every 100 atoms of natural copper, 69. 09 are 63 Cu and 30. 91 are 65 Cu Copyright © 2018 Cengage Learning. All Rights Reserved.

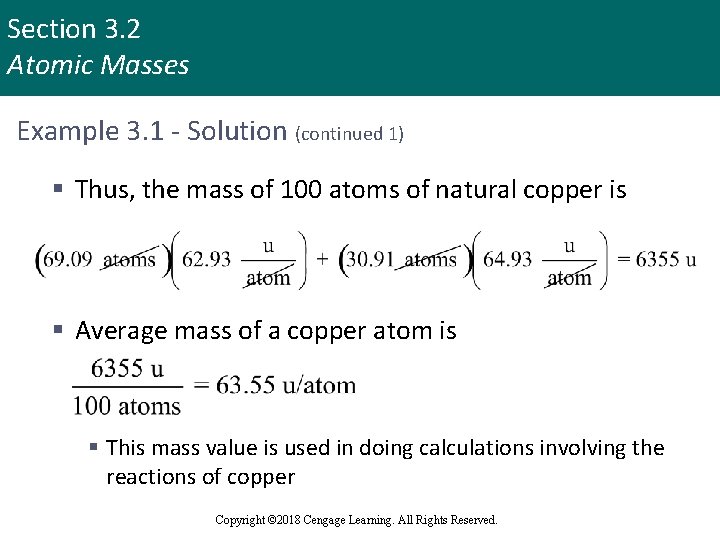
Section 3. 2 Atomic Masses Example 3. 1 - Solution (continued 1) § Thus, the mass of 100 atoms of natural copper is § Average mass of a copper atom is § This mass value is used in doing calculations involving the reactions of copper Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 2 Atomic Masses Example 3. 1 - Solution (continued 2) § Reality check § Answer (63. 55 u) is between the masses of the atoms that make up natural copper and makes sense § Answer could not be smaller than 62. 93 u or larger than 64. 93 u Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 2 Atomic Masses Join In (1) § The atomic number of Indium is 49 and its atomic mass 114. 8 g § Naturally occurring indium contains a mixture of indium-112 and indium-115, respectively, in an atomic ratio of approximately: Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 2 Atomic Masses Join In (1) (continued) a. b. c. d. e. 6: 94 25: 75 50: 50 75: 25 94: 6 Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 2 Atomic Masses Join In (2) § You have a sample of zinc (Zn) and a sample of aluminum (Al) § Each sample contains the same number of atoms § Which of the following statements concerning the masses of the samples is true? Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 2 Atomic Masses Join In (2) (continued) a. b. c. d. e. Mass of the zinc sample is more than twice as great as the mass of the aluminum sample Mass of the zinc sample is more than the mass of the aluminum sample, but it is not twice as great Mass of the aluminum sample is more than twice as great as the mass of the zinc sample Mass of the aluminum sample is more than the mass of the zinc sample, but it is not twice as great Masses of the two samples are equal Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 3 The Mole (mol) § Unit of measure established for use in counting atoms § Number equal to the number of carbon atoms in exactly 12 grams of pure 12 C § Techniques such as mass spectrometry determine this number to be 6. 02214 × 1023 § Avogadro’s number: One mole of something consists of 6. 022× 1023 units of that substance Copyright © Cengage Learning. All rights reserved Copyright © 2018 Cengage Learning. All Rights Reserved. 23

Chapter 3 Stoichiometry Copyright © 2018 Cengage Learning. All Rights Reserved.

Chapter 3 Stoichiometry Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 3 The Mole Critical Thinking § What if you were offered $1 million to count from 1 to 6 × 1023 at a rate of one number each second? § Determine your hourly wage § Would you do it? Could you do it? Copyright © 2018 Cengage Learning. All Rights Reserved.

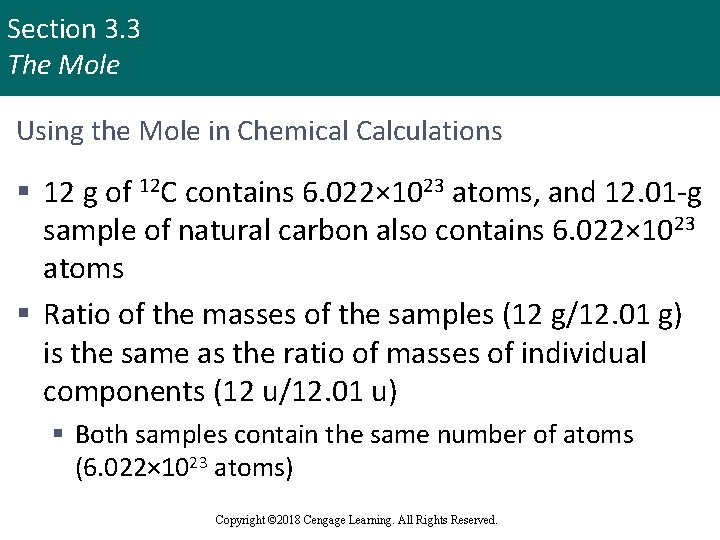
Section 3. 3 The Mole Using the Mole in Chemical Calculations § 12 g of 12 C contains 6. 022× 1023 atoms, and 12. 01 -g sample of natural carbon also contains 6. 022× 1023 atoms § Ratio of the masses of the samples (12 g/12. 01 g) is the same as the ratio of masses of individual components (12 u/12. 01 u) § Both samples contain the same number of atoms (6. 022× 1023 atoms) Copyright © 2018 Cengage Learning. All Rights Reserved.

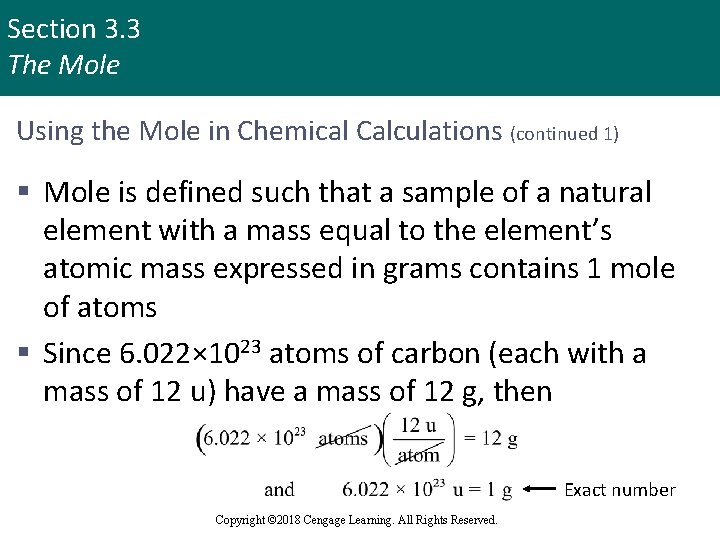
Section 3. 3 The Mole Using the Mole in Chemical Calculations (continued 1) § Mole is defined such that a sample of a natural element with a mass equal to the element’s atomic mass expressed in grams contains 1 mole of atoms § Since 6. 022× 1023 atoms of carbon (each with a mass of 12 u) have a mass of 12 g, then Exact number Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 3 The Mole Using the Mole in Chemical Calculations (continued 2) § Relationship can be used to derive the unit factor required to convert between atomic mass units and grams Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 3 The Mole Critical Thinking § What if you discovered Avogadro’s number was not 6. 02× 1023 but 3. 01× 1023? § Would this affect the relative masses given on the periodic table? § If so, how? § If not, why not? Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 3 The Mole Interactive Example 3. 5 - Calculating the Number of Moles and Mass § Cobalt (Co) is a metal that is added to steel to improve its resistance to corrosion § Calculate both the number of moles in a sample of cobalt containing 5. 00 × 1020 atoms and the mass of the sample Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 3 The Mole Interactive Example 3. 5 - Solution § Where are we going? § To calculate the number of moles and the mass of a sample of Co § What do we know? § Sample contains 5. 00 × 1020 atoms of Co Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 3 The Mole Interactive Example 3. 5 - Solution (continued 1) § How do we get there? § Note that the sample of 5. 00 × 1020 atoms of cobalt is less than 1 mole (6. 022 × 1023 atoms) of cobalt § What fraction of a mole it represents can be determined as follows: Copyright © 2018 Cengage Learning. All Rights Reserved.

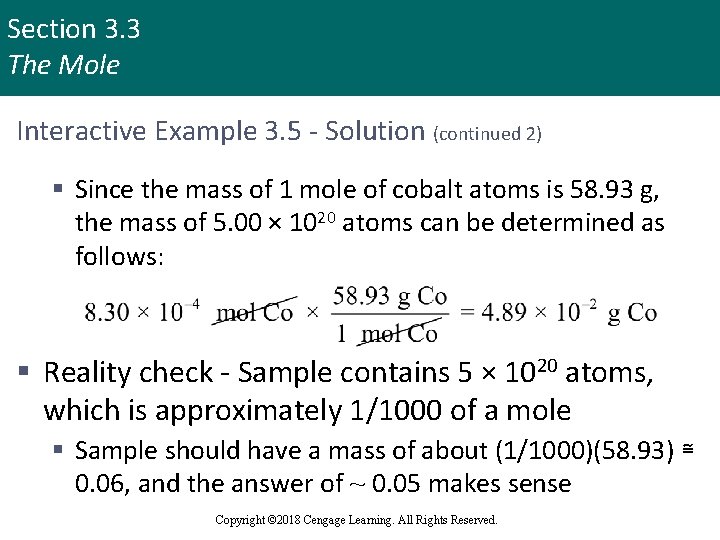
Section 3. 3 The Mole Interactive Example 3. 5 - Solution (continued 2) § Since the mass of 1 mole of cobalt atoms is 58. 93 g, the mass of 5. 00 × 1020 atoms can be determined as follows: § Reality check - Sample contains 5 × 1020 atoms, which is approximately 1/1000 of a mole § Sample should have a mass of about (1/1000)(58. 93) ≅ 0. 06, and the answer of ~ 0. 05 makes sense Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 3 The Mole Exercise § Diamond is a natural form of pure carbon § What number of atoms of carbon are in a 1. 00 -carat diamond (1. 00 carat = 0. 200 g)? 1. 00× 1022 atoms C Copyright © Cengage Learning. All rights reserved Copyright © 2018 Cengage Learning. All Rights Reserved. 35

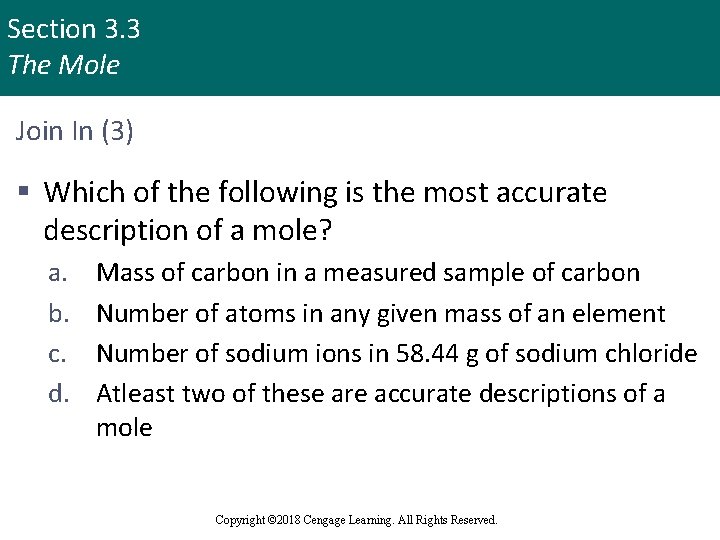
Section 3. 3 The Mole Join In (3) § Which of the following is the most accurate description of a mole? a. b. c. d. Mass of carbon in a measured sample of carbon Number of atoms in any given mass of an element Number of sodium ions in 58. 44 g of sodium chloride Atleast two of these are accurate descriptions of a mole Copyright © 2018 Cengage Learning. All Rights Reserved.

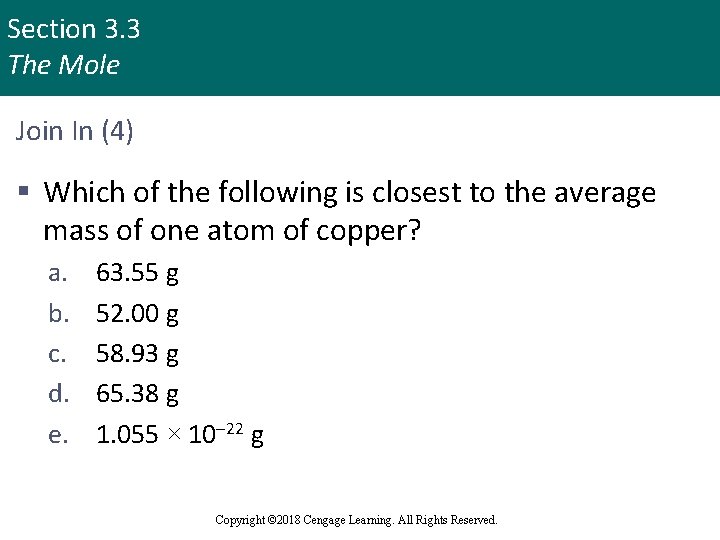
Section 3. 3 The Mole Join In (4) § Which of the following is closest to the average mass of one atom of copper? a. b. c. d. e. 63. 55 g 52. 00 g 58. 93 g 65. 38 g 1. 055 × 10– 22 g Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 3 The Mole Join In (5) § Which of the following 100. 0 -g samples contains the greatest number of atoms? a. b. c. d. e. Magnesium Zinc Silver Calcium All samples contain the same number of atoms Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 4 Molar Mass of a Substance § Mass in grams of one mole of the compound § Traditionally known as molecular weight § Obtained by summing the masses of the component atoms of a compound Copyright © Cengage Learning. All rights reserved Copyright © 2018 Cengage Learning. All Rights Reserved. 39

Section 3. 4 Molar Mass Ionic Substances § Contain simple ions and polyatomic ions § Fundamental unit of an ionic compound is called a formula unit § Example - Na. Cl is the formula unit for sodium chloride Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 4 Molar Mass Interactive Example 3. 7 - Calculating Molar Mass II § Calcium carbonate (Ca. CO 3), also called calcite, is the principal mineral found in limestone, marble, chalk, pearls, and the shells of marine animals such as clams a. Calculate the molar mass of calcium carbonate b. A certain sample of calcium carbonate contains 4. 86 moles § § What is the mass in grams of this sample? What is the mass of the CO 32– ions present? Copyright © 2018 Cengage Learning. All Rights Reserved.

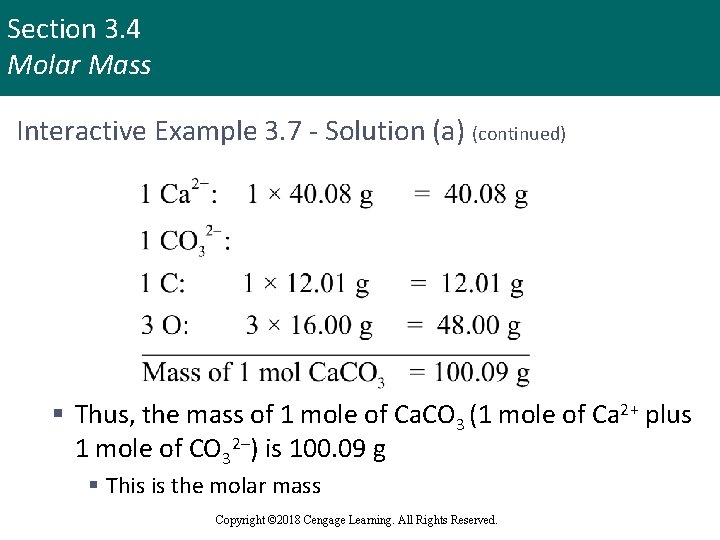
Section 3. 4 Molar Mass Interactive Example 3. 7 - Solution (a) § Calcium carbonate is an ionic compound composed of Ca 2+ and CO 32– ions § In 1 mole of calcium carbonate, there are 1 mole of Ca 2+ ions and 1 mole of CO 32– ions § Molar mass is calculated by summing the masses of the components Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 4 Molar Mass Interactive Example 3. 7 - Solution (a) (continued) § Thus, the mass of 1 mole of Ca. CO 3 (1 mole of Ca 2+ plus 1 mole of CO 32–) is 100. 09 g § This is the molar mass Copyright © 2018 Cengage Learning. All Rights Reserved.

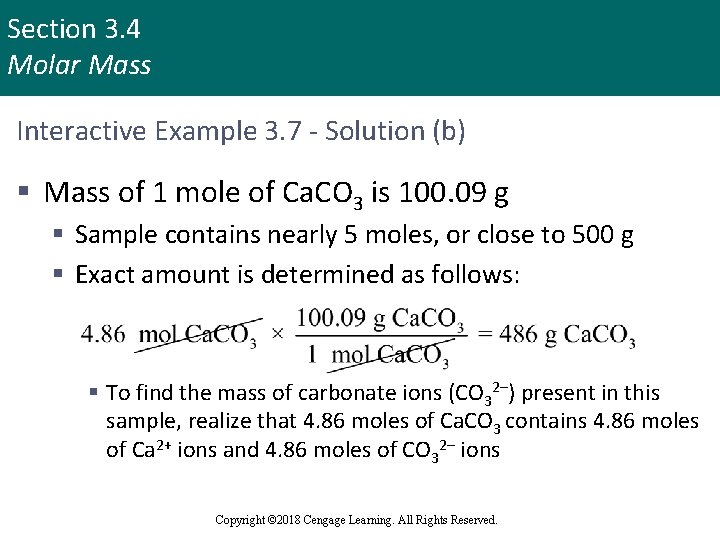
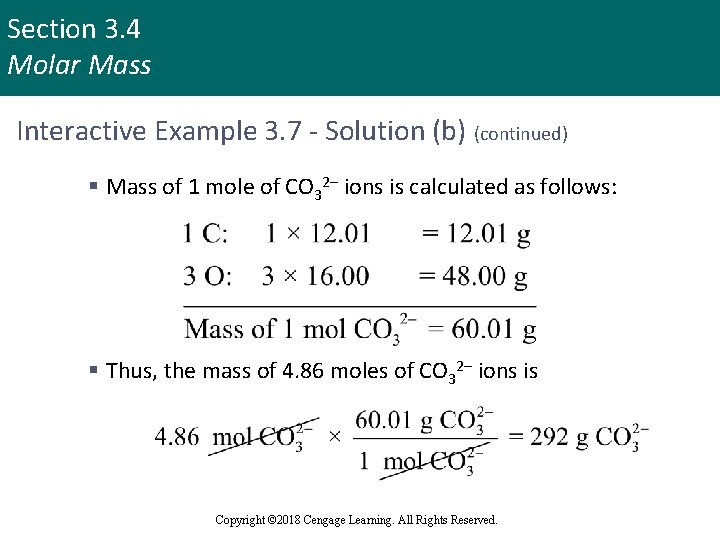
Section 3. 4 Molar Mass Interactive Example 3. 7 - Solution (b) § Mass of 1 mole of Ca. CO 3 is 100. 09 g § Sample contains nearly 5 moles, or close to 500 g § Exact amount is determined as follows: § To find the mass of carbonate ions (CO 32–) present in this sample, realize that 4. 86 moles of Ca. CO 3 contains 4. 86 moles of Ca 2+ ions and 4. 86 moles of CO 32– ions Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 4 Molar Mass Interactive Example 3. 7 - Solution (b) (continued) § Mass of 1 mole of CO 32– ions is calculated as follows: § Thus, the mass of 4. 86 moles of CO 32– ions is Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 4 Molar Mass Interactive Example 3. 8 - Molar Mass and Numbers of Molecules § Isopentyl acetate (C 7 H 14 O 2) is the compound responsible for the scent of bananas § Interestingly, bees release about 1 μg (1× 10– 6 g) of this compound when they sting § Resulting scent attracts other bees to join the attack § How many molecules of isopentyl acetate are released in a typical bee sting? § How many atoms of carbon are present? Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 4 Molar Mass Interactive Example 3. 8 - Solution § Where are we going? § To calculate the number of molecules of C 7 H 14 O 2 and the number of carbon atoms in a bee sting § What do we know? § Mass of C 7 H 14 O 2 in a typical bee sting is 1 microgram = 1× 10– 6 g Copyright © 2018 Cengage Learning. All Rights Reserved.

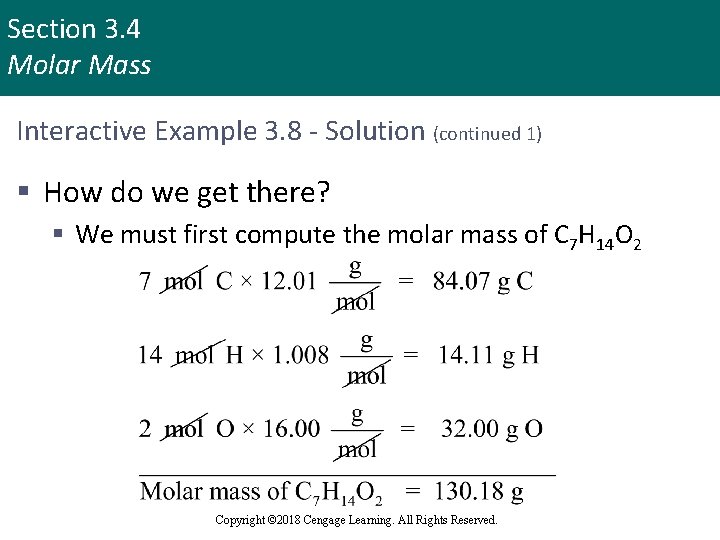
Section 3. 4 Molar Mass Interactive Example 3. 8 - Solution (continued 1) § How do we get there? § We must first compute the molar mass of C 7 H 14 O 2 Copyright © 2018 Cengage Learning. All Rights Reserved.

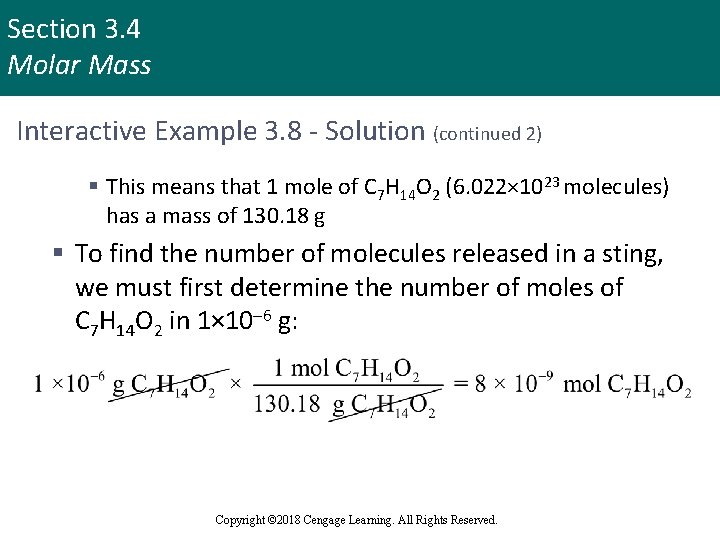
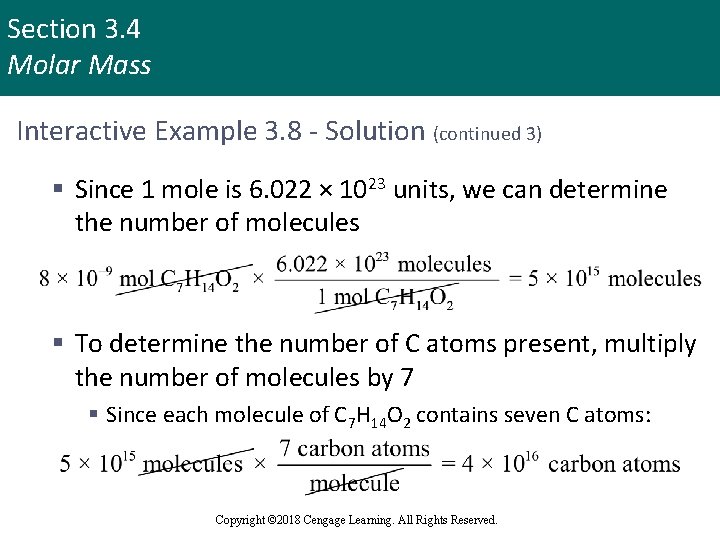
Section 3. 4 Molar Mass Interactive Example 3. 8 - Solution (continued 2) § This means that 1 mole of C 7 H 14 O 2 (6. 022× 1023 molecules) has a mass of 130. 18 g § To find the number of molecules released in a sting, we must first determine the number of moles of C 7 H 14 O 2 in 1× 10– 6 g: Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 4 Molar Mass Interactive Example 3. 8 - Solution (continued 3) § Since 1 mole is 6. 022 × 1023 units, we can determine the number of molecules § To determine the number of C atoms present, multiply the number of molecules by 7 § Since each molecule of C 7 H 14 O 2 contains seven C atoms: Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 4 Molar Mass Interactive Example 3. 8 - Solution (continued 4) § Note § In keeping with our practice of always showing the correct number of significant figures, we have rounded after each step § However, if extra digits are carried throughout this problem, the final answer rounds to 3× 1016 Copyright © 2018 Cengage Learning. All Rights Reserved.

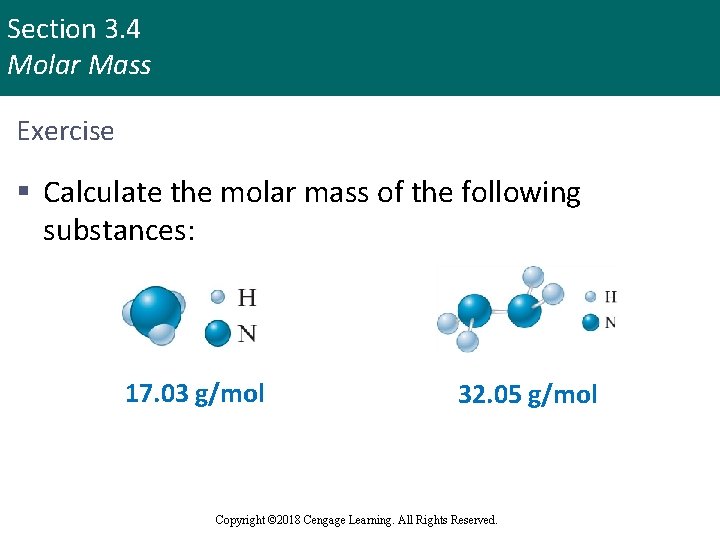
Section 3. 4 Molar Mass Exercise § Calculate the molar mass of the following substances: 17. 03 g/mol Copyright © Cengage Learning. All rights reserved 32. 05 g/mol Copyright © 2018 Cengage Learning. All Rights Reserved. 52

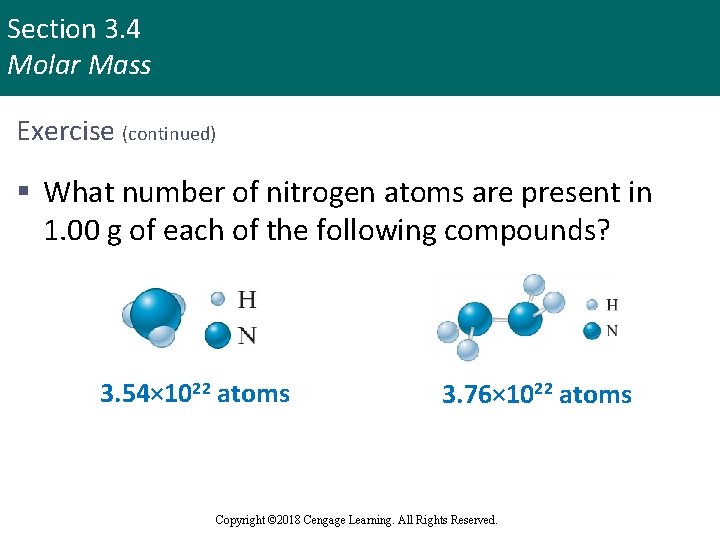
Section 3. 4 Molar Mass Exercise (continued) § What number of nitrogen atoms are present in 1. 00 g of each of the following compounds? 3. 54× 1022 atoms Copyright © Cengage Learning. All rights reserved 3. 76× 1022 atoms Copyright © 2018 Cengage Learning. All Rights Reserved. 53

Section 3. 4 Molar Mass Join In (6) § For which of the following compounds does 1. 0 g represent 2. 27 10– 2 mol? a. b. c. d. H 2 O CO 2 NH 3 C 2 H 6 Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 4 Molar Mass Join In (7) § Mass of 0. 82 mol of a diatomic molecule is 131. 3 g § Identify the molecule a. F 2 b. Cl 2 c. Br 2 d. I 2 Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 4 Molar Mass Join In (8) § Which of the following 100. 0 -g samples contains the greatest number of oxygen atoms? a. b. c. d. e. H 2 O N 2 O C 3 H 6 O 2 CO 2 All of the samples have the same number of oxygen atoms Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 5 Learning to Solve Problems Conceptual Problem Solving § Method that helps solve problems in a flexible and creative manner based on the understanding of fundamental concepts of chemistry § Goal of the text § To help individuals solve new problems on their own Copyright © Cengage Learning. All rights reserved Copyright © 2018 Cengage Learning. All Rights Reserved. 57

Section 3. 5 Learning to Solve Problems Methods of Approaching a Problem § Pigeonholing method § Emphasizes memorization § Involves labeling the problem § Slotting the problem into the pigeonhole that fits best § Provides steps that one can memorize and store in an appropriate slot for each different problem § Challenge - Requires a new pigeonhole for every new problem Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 5 Learning to Solve Problems Methods of Approaching a Problem (continued) § Conceptual problem solving § Helps understand the big picture § Involves looking for a solution within the problem § Each problem is assumed as a new one § Involves asking a series of questions § One uses his/her knowledge of fundamental principles of chemistry to answer the questions Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 5 Learning to Solve Problems Understanding Conceptual Problem Solving § Where are we going? § Read the problem and decide on the final goal § Sort through the given facts and focus on the key words § Draw a diagram of the problem § This stage involves a simple, visual analysis of the problem Copyright © Cengage Learning. All rights reserved Copyright © 2018 Cengage Learning. All Rights Reserved. 60

Section 3. 5 Learning to Solve Problems Understanding Conceptual Problem Solving (continued) § How do we get there? § Involves working backward from the final goal in order to decide the starting point § Understanding of the fundamental principles can help lead to the final solution § Reality check § Involves checking if the answer makes sense and whether the answer is reasonable Copyright © Cengage Learning. All rights reserved Copyright © 2018 Cengage Learning. All Rights Reserved. 61

Section 3. 6 Percent Composition of Compounds Methods of Describing a Compound’s Composition § In terms of the numbers of the compound’s constituent atoms § In terms of mass percent (weight percent) Copyright © Cengage Learning. All rights reserved Copyright © 2018 Cengage Learning. All Rights Reserved. 62

Section 3. 6 Percent Composition of Compounds Interactive Example 3. 9 - Calculating Mass Percent § Carvone is a substance that occurs in two forms having different arrangements of the atoms but the same molecular formula (C 10 H 14 O) and mass § One type of carvone gives caraway seeds their characteristic smell, and the other type is responsible for the smell of spearmint oil § Compute the mass percent of each element in carvone Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 6 Percent Composition of Compounds Interactive Example 3. 9 - Solution § Where are we going? § To find the mass percent of each element in carvone § What do we know? § Molecular formula is C 10 H 14 O § What information do we need to find the mass percent? § Mass of each element (we’ll use 1 mole of carvone) § Molar mass of carvone Copyright © 2018 Cengage Learning. All Rights Reserved.

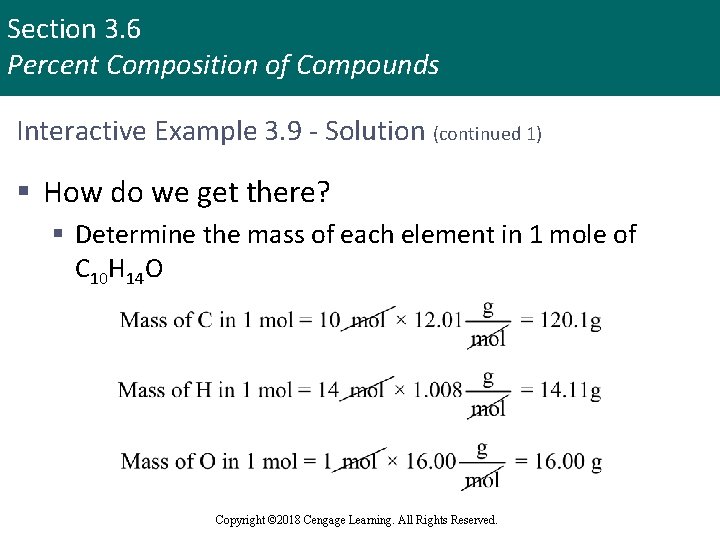
Section 3. 6 Percent Composition of Compounds Interactive Example 3. 9 - Solution (continued 1) § How do we get there? § Determine the mass of each element in 1 mole of C 10 H 14 O Copyright © 2018 Cengage Learning. All Rights Reserved.

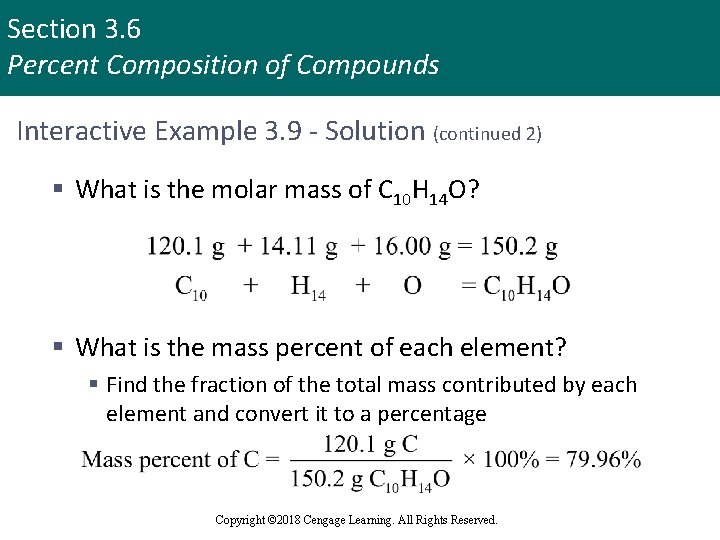
Section 3. 6 Percent Composition of Compounds Interactive Example 3. 9 - Solution (continued 2) § What is the molar mass of C 10 H 14 O? § What is the mass percent of each element? § Find the fraction of the total mass contributed by each element and convert it to a percentage Copyright © 2018 Cengage Learning. All Rights Reserved.

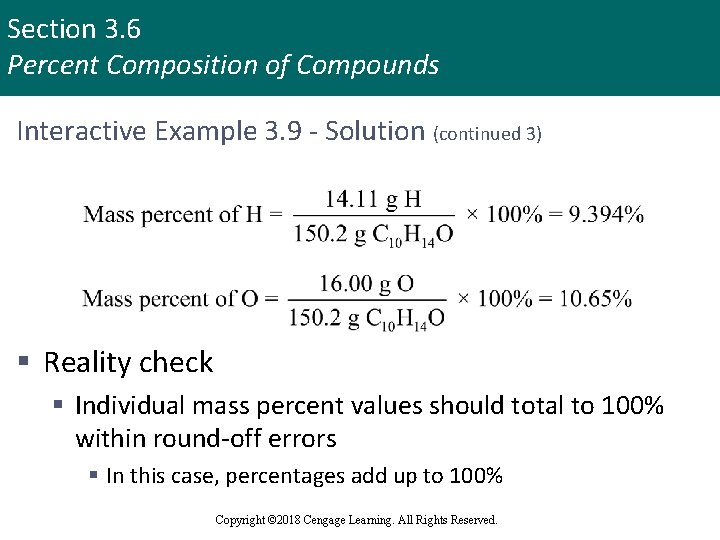
Section 3. 6 Percent Composition of Compounds Interactive Example 3. 9 - Solution (continued 3) § Reality check § Individual mass percent values should total to 100% within round-off errors § In this case, percentages add up to 100% Copyright © 2018 Cengage Learning. All Rights Reserved.

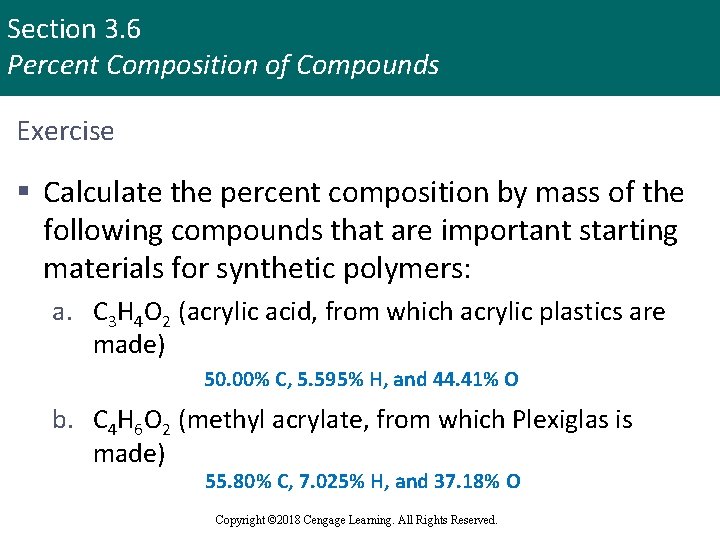
Section 3. 6 Percent Composition of Compounds Exercise § Calculate the percent composition by mass of the following compounds that are important starting materials for synthetic polymers: a. C 3 H 4 O 2 (acrylic acid, from which acrylic plastics are made) 50. 00% C, 5. 595% H, and 44. 41% O b. C 4 H 6 O 2 (methyl acrylate, from which Plexiglas is made) 55. 80% C, 7. 025% H, and 37. 18% O Copyright © Cengage Learning. All rights reserved Copyright © 2018 Cengage Learning. All Rights Reserved. 68

Section 3. 6 Percent Composition of Compounds Join In (9) § Which of the following 100. 0 -g samples contains the highest percent oxygen by mass? a. b. c. d. e. H 2 O N 2 O C 3 H 6 O 2 CO 2 All of the samples have the same percent oxygen by mass Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 7 Determining the Formula of a Compound - Process § Weigh the sample of the compound by: § Decomposing the sample into its constituent elements § Reacting it with oxygen § Resulting substances are then collected and weighed § This type of analysis is done by a combustion device § Results of such analyses provide the mass of each type of element in the compound, which can be used to determine the mass percent of each element Copyright © Cengage Learning. All rights reserved Copyright © 2018 Cengage Learning. All Rights Reserved. 70

Chapter 3 Stoichiometry Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 7 Determining the Formula of a Compound Empirical Formula § Any molecule that can be represented as (CH 5 N)n has the empirical formula CH 5 N § n - Integer § Molecular formula: Exact formula of the molecules present in a substance § Molecular formula = (empirical formula)n § Requires the knowledge of the molar mass Copyright © Cengage Learning. All rights reserved Copyright © 2018 Cengage Learning. All Rights Reserved. 72

Section 3. 7 Determining the Formula of a Compound Problem-Solving Strategy - Empirical Formula Determination § Base the calculation on 100 g of compound as mass percentage gives the number of grams of a particular element per 100 g of compound § Each percent will then represent the mass in grams of that element § Determine the number of moles of each element present in 100 g of compound using the atomic masses of the elements present Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 7 Determining the Formula of a Compound Problem-Solving Strategy - Empirical Formula Determination (continued) § Divide each value of the number of moles by the smallest of the values § If each resulting number is a whole number (after appropriate rounding), these numbers represent the subscripts of the elements in the empirical formula § If the numbers obtained are not whole numbers, multiply each number by an integer so that the results are all whole numbers Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 7 Determining the Formula of a Compound Critical Thinking § One part of the problem-solving strategy for empirical formula determination is to base the calculation on 100 g of compound § What if you chose a mass other than 100 g? § Would this work? § What if you chose to base the calculation on 100 moles of compound? § Would this work? Copyright © 2018 Cengage Learning. All Rights Reserved.

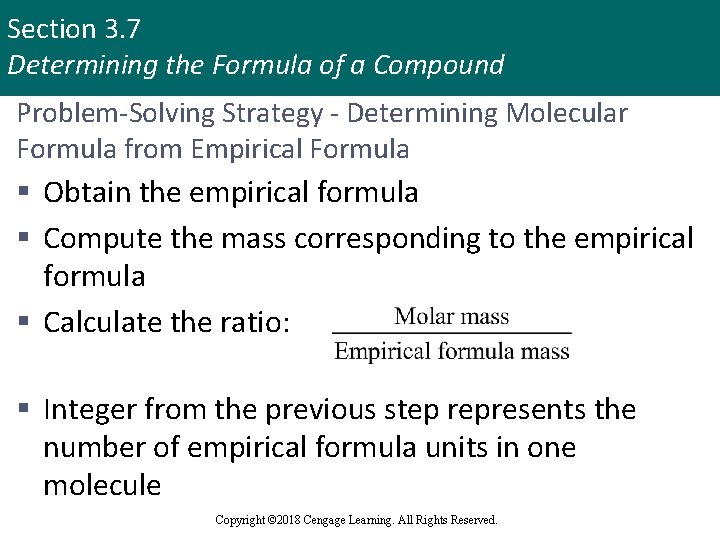
Section 3. 7 Determining the Formula of a Compound Problem-Solving Strategy - Determining Molecular Formula from Empirical Formula § Obtain the empirical formula § Compute the mass corresponding to the empirical formula § Calculate the ratio: § Integer from the previous step represents the number of empirical formula units in one molecule Copyright © 2018 Cengage Learning. All Rights Reserved.

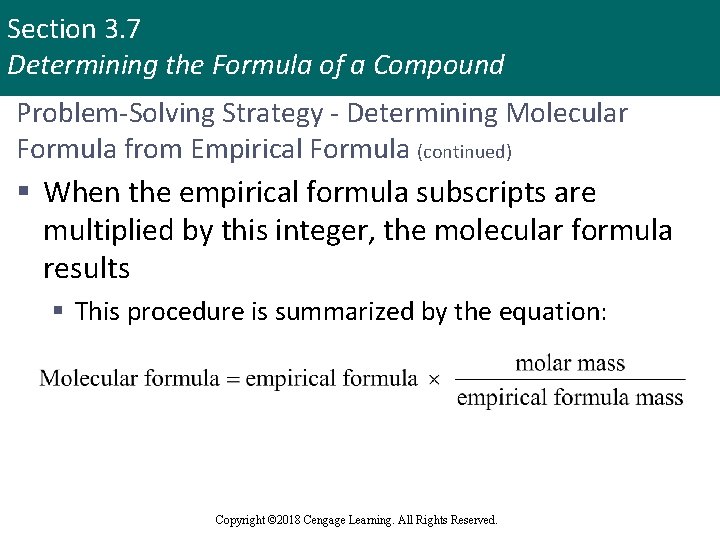
Section 3. 7 Determining the Formula of a Compound Problem-Solving Strategy - Determining Molecular Formula from Empirical Formula (continued) § When the empirical formula subscripts are multiplied by this integer, the molecular formula results § This procedure is summarized by the equation: Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 7 Determining the Formula of a Compound Interactive Example 3. 11 - Determining Empirical and Molecular Formulas II § A white powder is analyzed and found to contain 43. 64% phosphorus and 56. 36% oxygen by mass § Compound has a molar mass of 283. 88 g/mol § What are the compound’s empirical and molecular formulas? Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 7 Determining the Formula of a Compound Interactive Example 3. 11 - Solution § Where are we going? § To find the empirical and molecular formulas for the given compound § What do we know? § Percent of each element § Molar mass of the compound is 283. 88 g/mol Copyright © 2018 Cengage Learning. All Rights Reserved.

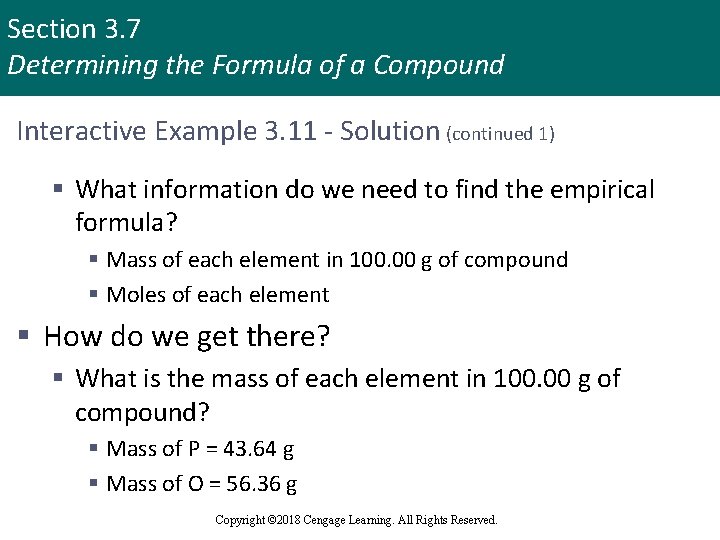
Section 3. 7 Determining the Formula of a Compound Interactive Example 3. 11 - Solution (continued 1) § What information do we need to find the empirical formula? § Mass of each element in 100. 00 g of compound § Moles of each element § How do we get there? § What is the mass of each element in 100. 00 g of compound? § Mass of P = 43. 64 g § Mass of O = 56. 36 g Copyright © 2018 Cengage Learning. All Rights Reserved.

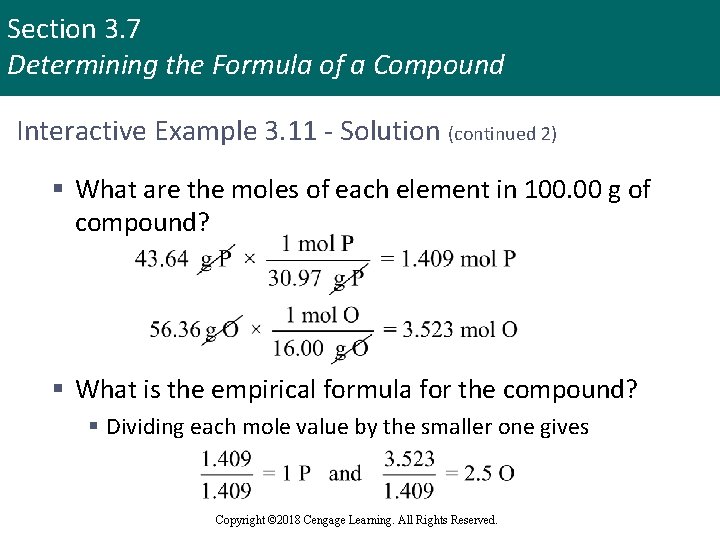
Section 3. 7 Determining the Formula of a Compound Interactive Example 3. 11 - Solution (continued 2) § What are the moles of each element in 100. 00 g of compound? § What is the empirical formula for the compound? § Dividing each mole value by the smaller one gives Copyright © 2018 Cengage Learning. All Rights Reserved.

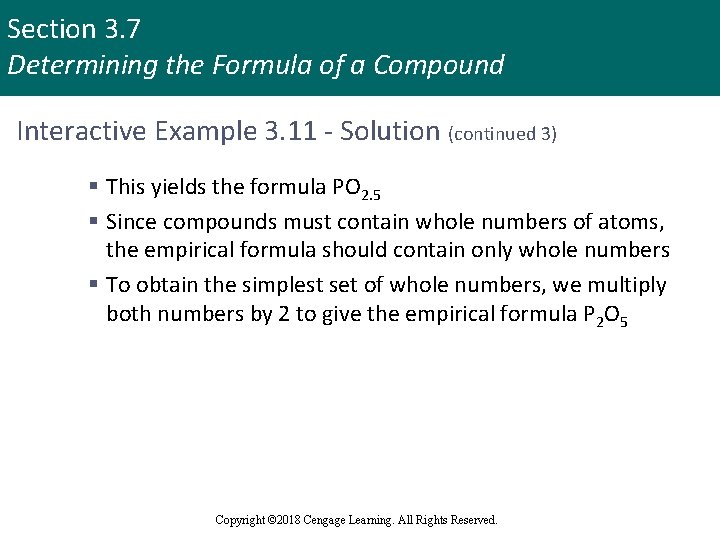
Section 3. 7 Determining the Formula of a Compound Interactive Example 3. 11 - Solution (continued 3) § This yields the formula PO 2. 5 § Since compounds must contain whole numbers of atoms, the empirical formula should contain only whole numbers § To obtain the simplest set of whole numbers, we multiply both numbers by 2 to give the empirical formula P 2 O 5 Copyright © 2018 Cengage Learning. All Rights Reserved.

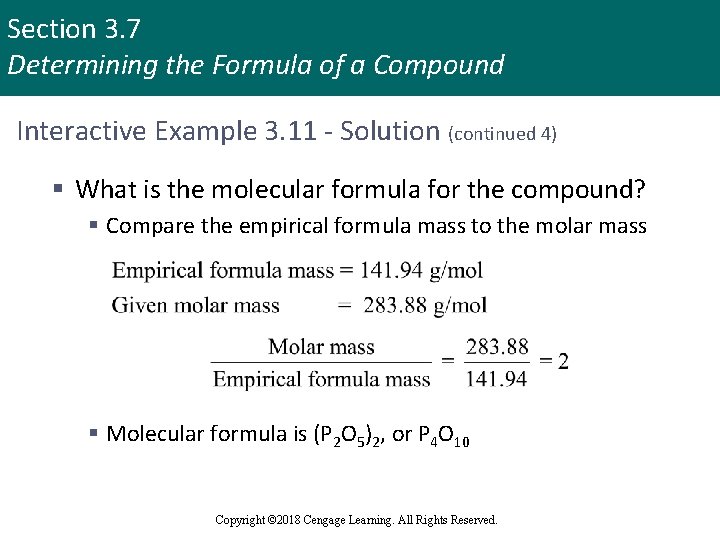
Section 3. 7 Determining the Formula of a Compound Interactive Example 3. 11 - Solution (continued 4) § What is the molecular formula for the compound? § Compare the empirical formula mass to the molar mass § Molecular formula is (P 2 O 5)2, or P 4 O 10 Copyright © 2018 Cengage Learning. All Rights Reserved.

Chapter 3 Stoichiometry Copyright © 2018 Cengage Learning. All Rights Reserved.

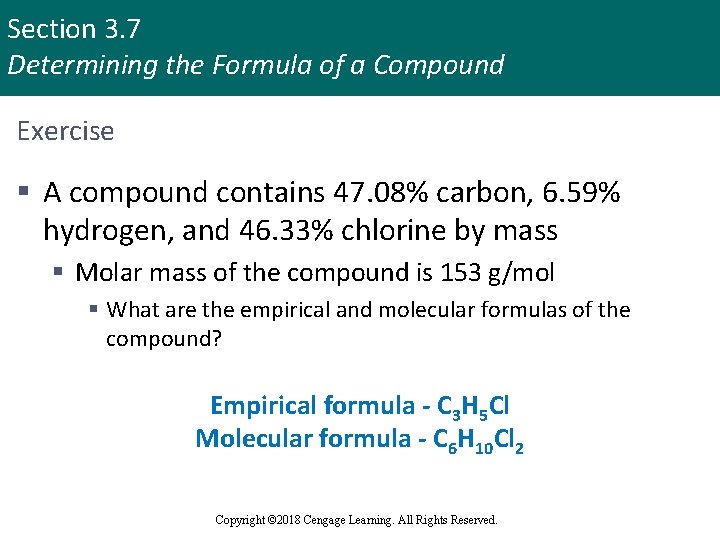
Section 3. 7 Determining the Formula of a Compound Exercise § A compound contains 47. 08% carbon, 6. 59% hydrogen, and 46. 33% chlorine by mass § Molar mass of the compound is 153 g/mol § What are the empirical and molecular formulas of the compound? Empirical formula - C 3 H 5 Cl Molecular formula - C 6 H 10 Cl 2 Copyright © Cengage Learning. All rights reserved Copyright © 2018 Cengage Learning. All Rights Reserved. 85

Section 3. 7 Determining the Formula of a Compound Problem-Solving Strategy - Determining Molecular Formula from Mass Percent and Molar Mass § Using the mass percentages and the molar mass, determine the mass of each element present in 1 mole of compound § Determine the number of moles of each element present in 1 mole of compound § Integers in this step represent the subscripts in the molecular formula Copyright © 2018 Cengage Learning. All Rights Reserved.

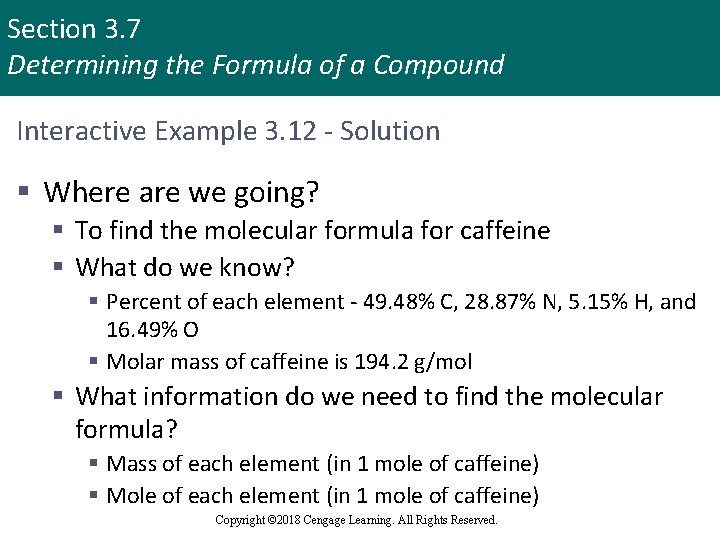
Section 3. 7 Determining the Formula of a Compound Interactive Example 3. 12 - Determining a Molecular Formula § Caffeine, a stimulant found in coffee, tea, and chocolate, contains 49. 48% carbon, 5. 15% hydrogen, 28. 87% nitrogen, and 16. 49% oxygen by mass and has a molar mass of 194. 2 g/mol § Determine the molecular formula of caffeine Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 7 Determining the Formula of a Compound Interactive Example 3. 12 - Solution § Where are we going? § To find the molecular formula for caffeine § What do we know? § Percent of each element - 49. 48% C, 28. 87% N, 5. 15% H, and 16. 49% O § Molar mass of caffeine is 194. 2 g/mol § What information do we need to find the molecular formula? § Mass of each element (in 1 mole of caffeine) § Mole of each element (in 1 mole of caffeine) Copyright © 2018 Cengage Learning. All Rights Reserved.

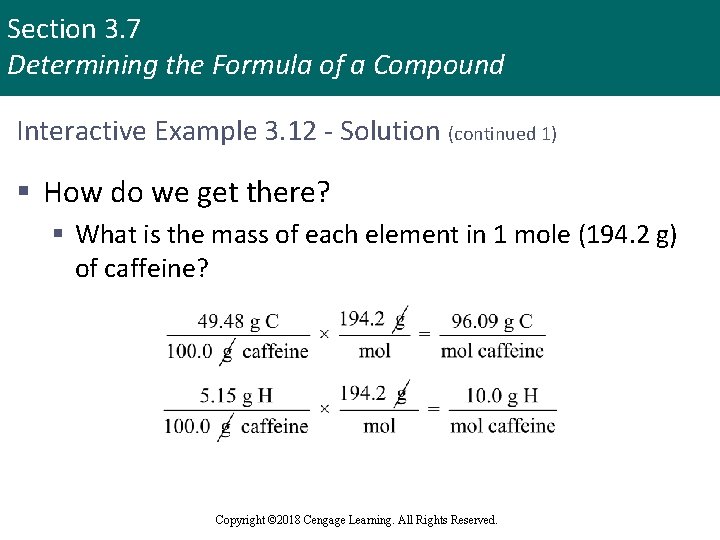
Section 3. 7 Determining the Formula of a Compound Interactive Example 3. 12 - Solution (continued 1) § How do we get there? § What is the mass of each element in 1 mole (194. 2 g) of caffeine? Copyright © 2018 Cengage Learning. All Rights Reserved.

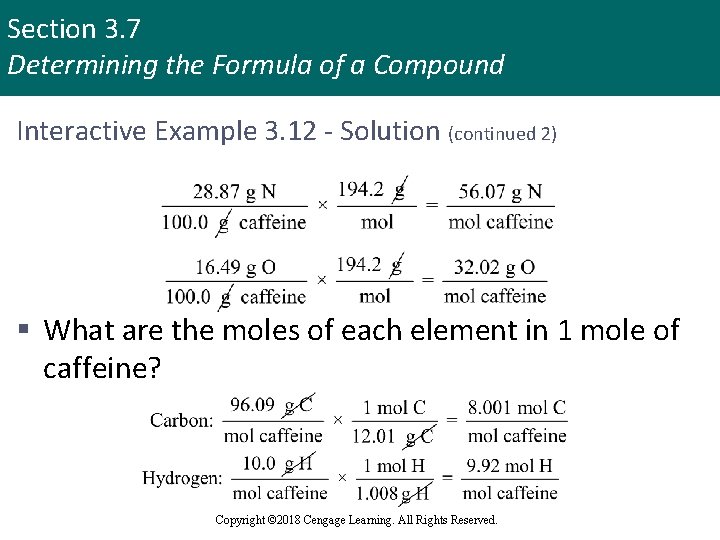
Section 3. 7 Determining the Formula of a Compound Interactive Example 3. 12 - Solution (continued 2) § What are the moles of each element in 1 mole of caffeine? Copyright © 2018 Cengage Learning. All Rights Reserved.

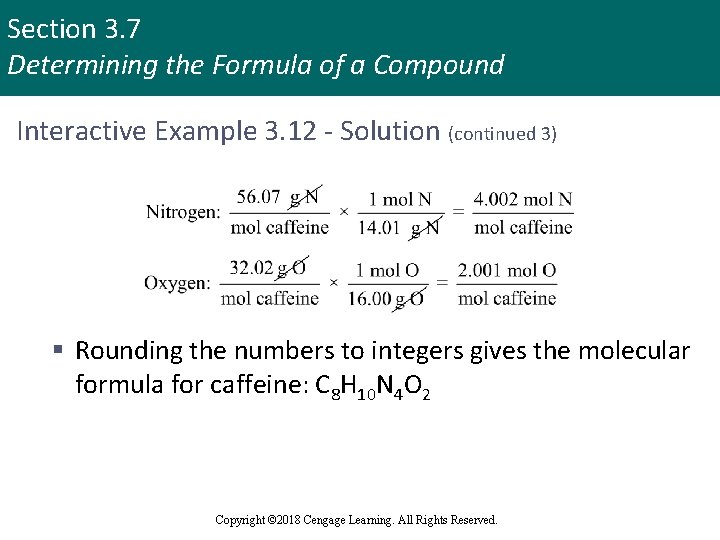
Section 3. 7 Determining the Formula of a Compound Interactive Example 3. 12 - Solution (continued 3) § Rounding the numbers to integers gives the molecular formula for caffeine: C 8 H 10 N 4 O 2 Copyright © 2018 Cengage Learning. All Rights Reserved.

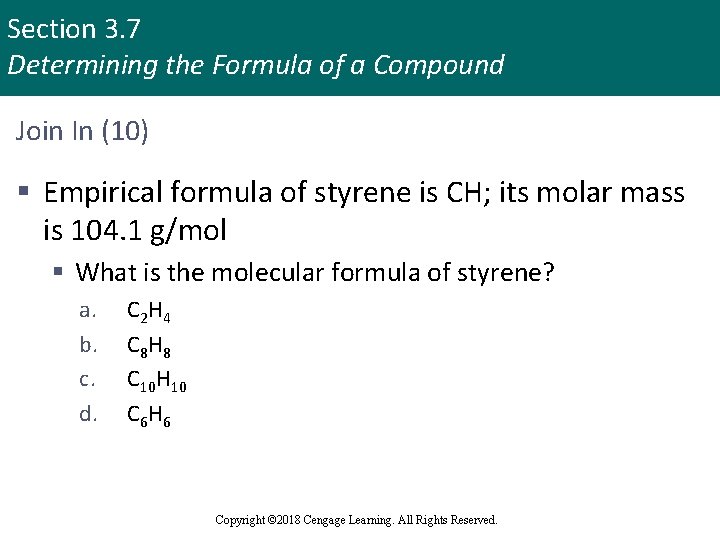
Section 3. 7 Determining the Formula of a Compound Join In (10) § Empirical formula of styrene is CH; its molar mass is 104. 1 g/mol § What is the molecular formula of styrene? a. b. c. d. C 2 H 4 C 8 H 8 C 10 H 10 C 6 H 6 Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 8 Chemical Equations Chemical Change § Involves the reorganization of atoms in one or more substances § Atoms are neither created nor destroyed § Represented by a chemical equation § Reactants: Presented on the left side of an arrow § Products: Presented on the right side of the arrow Reactants Copyright © Cengage Learning. All rights reserved Products Copyright © 2018 Cengage Learning. All Rights Reserved. 93

Section 3. 8 Chemical Equations Balancing a Chemical Equation § All atoms present in the reactants must be accounted for among the products § Same number of each type of atom should be present on the products side and on the reactants side of the arrow Copyright © 2018 Cengage Learning. All Rights Reserved.

Chapter 3 Stoichiometry Copyright © 2018 Cengage Learning. All Rights Reserved.

Chapter 3 Stoichiometry Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 8 Chemical Equations Information Provided by Chemical Equations (continued) § Relative numbers of reactants and products § Indicated by coefficients in a balanced equation § Mass remains constant § Atoms are conserved in a chemical reaction Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 9 Balancing Chemical Equations Things to Remember § An unbalanced equation is of limited use § Identities of the reactants and products of a reaction must be recognized by experimental observation § Formulas of compounds must never be changed while balancing a chemical equation § Subscripts in a formula cannot be changed, nor can atoms be added or subtracted from a formula Copyright © Cengage Learning. All rights reserved Copyright © 2018 Cengage Learning. All Rights Reserved. 98

Section 3. 9 Balancing Chemical Equations Critical Thinking § What if a friend was balancing chemical equations by changing the values of the subscripts instead of using the coefficients? § How would you explain to your friend that this was the wrong thing to do? Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 9 Balancing Chemical Equations Problem-Solving Strategy - Writing and Balancing the Equation for a Chemical Reaction 1. Determine what reaction is occurring § Determine the reactants, the products, and the physical states involved 2. Write the unbalanced equation that summarizes the reaction Copyright © Cengage Learning. All rights reserved Copyright © 2018 Cengage Learning. All Rights Reserved. 100

Section 3. 9 Balancing Chemical Equations Problem-Solving Strategy - Writing and Balancing the Equation for a Chemical Reaction (continued) 3. Balance the equation by inspection, starting with the most complicated molecule(s) § Determine what coefficients are necessary § Same number of each type of atom needs to appear on both reactant and product sides § Do not change the formulas of any of the reactants or products Copyright © Cengage Learning. All rights reserved Copyright © 2018 Cengage Learning. All Rights Reserved. 101

Section 3. 9 Balancing Chemical Equations Critical Thinking § One part of the problem-solving strategy for balancing chemical equations is starting with the most complicated molecule § What if you started with a different molecule? § Could you still eventually balance the chemical equation? § How would this approach be different from the suggested technique? Copyright © 2018 Cengage Learning. All Rights Reserved.

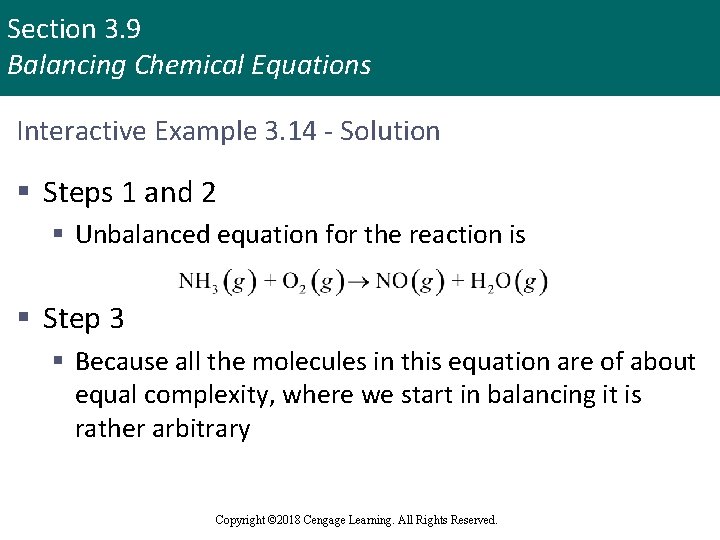
Section 3. 9 Balancing Chemical Equations Interactive Example 3. 14 - Balancing a Chemical Equation II § At 1000°C, ammonia gas, NH 3(g), reacts with oxygen gas to form gaseous nitric oxide, NO(g), and water vapor § This reaction is the first step in the commercial production of nitric acid by the Ostwald process § Balance the equation for this reaction Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 9 Balancing Chemical Equations Interactive Example 3. 14 - Solution § Steps 1 and 2 § Unbalanced equation for the reaction is § Step 3 § Because all the molecules in this equation are of about equal complexity, where we start in balancing it is rather arbitrary Copyright © 2018 Cengage Learning. All Rights Reserved.

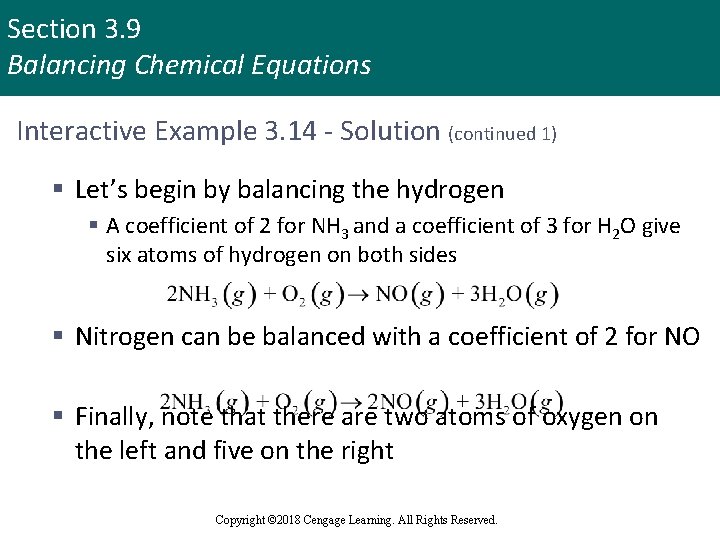
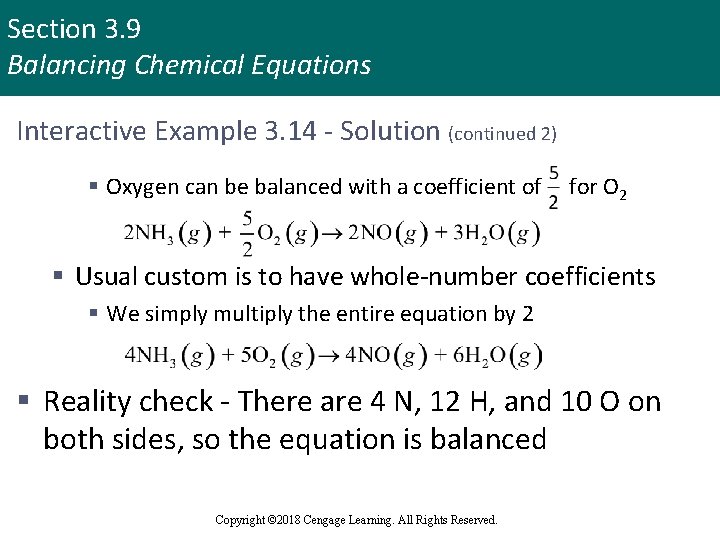
Section 3. 9 Balancing Chemical Equations Interactive Example 3. 14 - Solution (continued 1) § Let’s begin by balancing the hydrogen § A coefficient of 2 for NH 3 and a coefficient of 3 for H 2 O give six atoms of hydrogen on both sides § Nitrogen can be balanced with a coefficient of 2 for NO § Finally, note that there are two atoms of oxygen on the left and five on the right Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 9 Balancing Chemical Equations Interactive Example 3. 14 - Solution (continued 2) § Oxygen can be balanced with a coefficient of for O 2 § Usual custom is to have whole-number coefficients § We simply multiply the entire equation by 2 § Reality check - There are 4 N, 12 H, and 10 O on both sides, so the equation is balanced Copyright © 2018 Cengage Learning. All Rights Reserved.

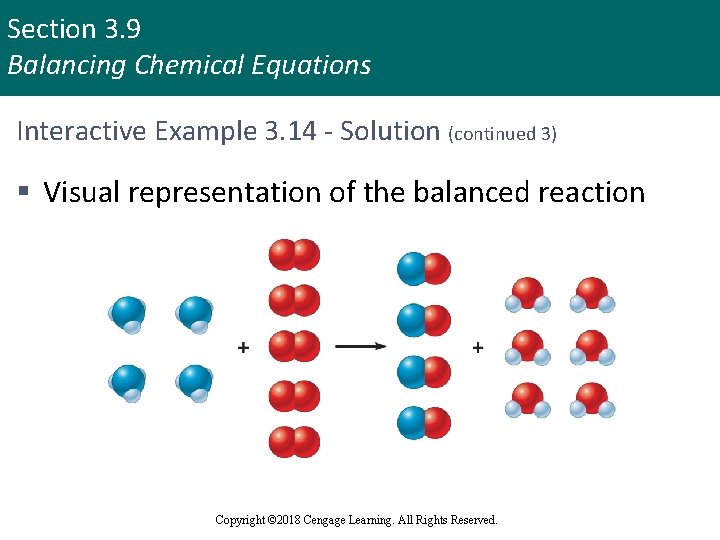
Section 3. 9 Balancing Chemical Equations Interactive Example 3. 14 - Solution (continued 3) § Visual representation of the balanced reaction Copyright © 2018 Cengage Learning. All Rights Reserved.

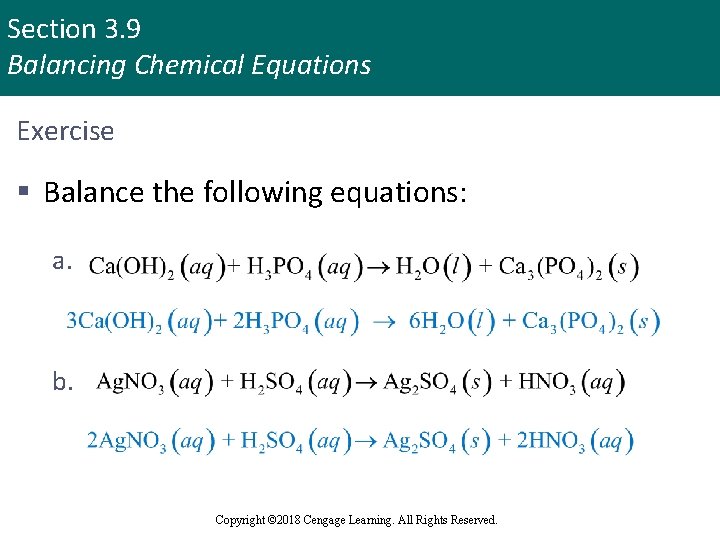
Section 3. 9 Balancing Chemical Equations Exercise § Balance the following equations: a. b. Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 9 Balancing Chemical Equations Join In (11) § When the equation NH 3 + O 2 NO + H 2 O is balanced with the smallest set of integers, the sum of the coefficients is a. b. c. d. e. 4 12 14 19 24 Copyright © 2018 Cengage Learning. All Rights Reserved.

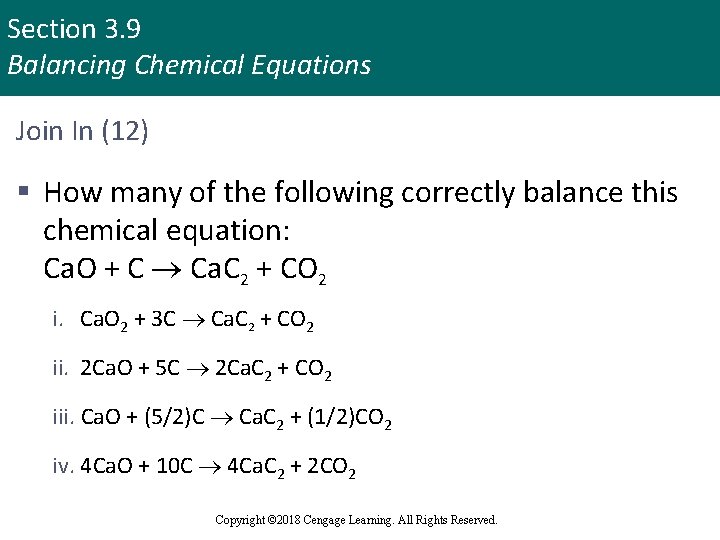
Section 3. 9 Balancing Chemical Equations Join In (12) § How many of the following correctly balance this chemical equation: Ca. O + C Ca. C 2 + CO 2 i. Ca. O 2 + 3 C Ca. C 2 + CO 2 ii. 2 Ca. O + 5 C 2 Ca. C 2 + CO 2 iii. Ca. O + (5/2)C Ca. C 2 + (1/2)CO 2 iv. 4 Ca. O + 10 C 4 Ca. C 2 + 2 CO 2 Copyright © 2018 Cengage Learning. All Rights Reserved.

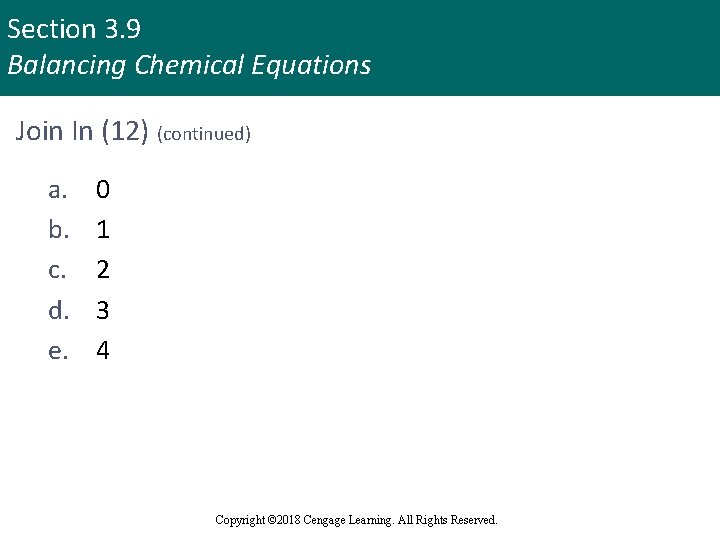
Section 3. 9 Balancing Chemical Equations Join In (12) (continued) a. b. c. d. e. 0 1 2 3 4 Copyright © 2018 Cengage Learning. All Rights Reserved.

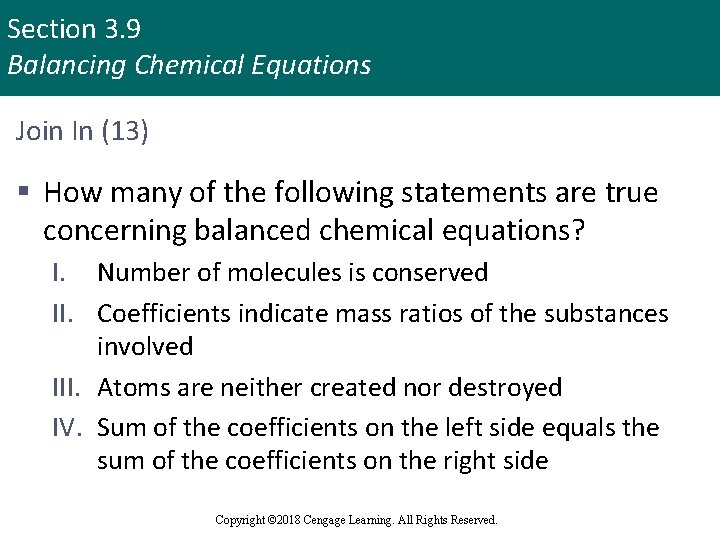
Section 3. 9 Balancing Chemical Equations Join In (13) § How many of the following statements are true concerning balanced chemical equations? I. Number of molecules is conserved II. Coefficients indicate mass ratios of the substances involved III. Atoms are neither created nor destroyed IV. Sum of the coefficients on the left side equals the sum of the coefficients on the right side Copyright © 2018 Cengage Learning. All Rights Reserved.

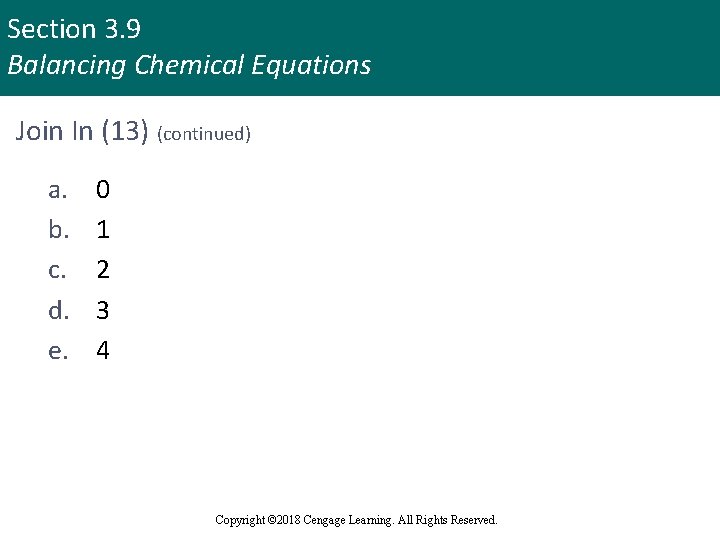
Section 3. 9 Balancing Chemical Equations Join In (13) (continued) a. b. c. d. e. 0 1 2 3 4 Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 10 Stoichiometric Calculations: Amounts of Reactants and Products Problem-Solving Strategy - Calculating Masses of Reactants and Products in Reactions § Balance the equation for the reaction § Convert the known mass of the reactant or product to moles of that substance § Use the balanced equation to set up the appropriate mole ratios § Use the appropriate mole ratios to calculate the number of moles of the desired reactant or product Copyright © Cengage Learning. All rights reserved Copyright © 2018 Cengage Learning. All Rights Reserved. 114

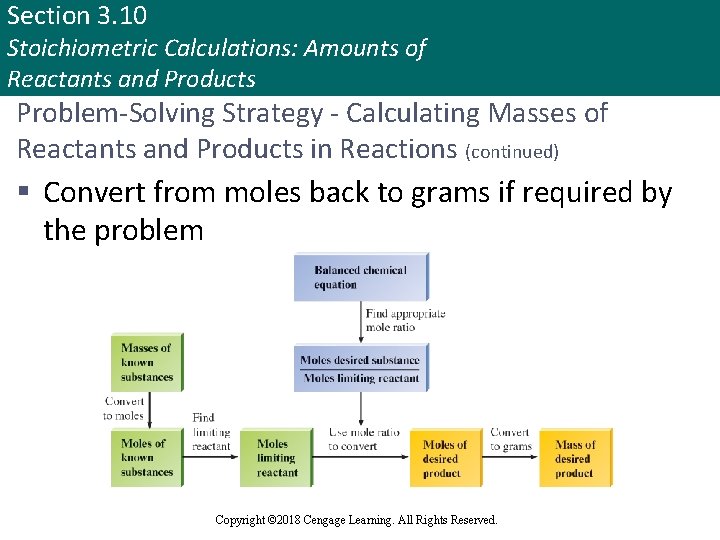
Section 3. 10 Stoichiometric Calculations: Amounts of Reactants and Products Problem-Solving Strategy - Calculating Masses of Reactants and Products in Reactions (continued) § Convert from moles back to grams if required by the problem Copyright © Cengage Learning. All rights reserved Copyright © 2018 Cengage Learning. All Rights Reserved. 115

Section 3. 10 Stoichiometric Calculations: Amounts of Reactants and Products Critical Thinking § Your lab partner observed that you always take the mass of chemicals in lab, but then you use mole ratios to balance the equation § “Why not use the masses in the equation? ” your partner asks § What if your lab partner decided to balance equations by using masses as coefficients? § Is this even possible? § Why or why not? Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 10 Stoichiometric Calculations: Amounts of Reactants and Products Interactive Example 3. 15 - Chemical Stoichiometry I § Solid lithium hydroxide is used in space vehicles to remove exhaled carbon dioxide from the living environment by forming solid lithium carbonate and liquid water § What mass of gaseous carbon dioxide can be absorbed by 1. 00 kg of lithium hydroxide? Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 10 Stoichiometric Calculations: Amounts of Reactants and Products Interactive Example 3. 15 - Solution § Where are we going? § To find the mass of CO 2 absorbed by 1. 00 kg Li. OH § What do we know? § Chemical reaction § 1. 00 kg Li. OH § What information is needed to find the mass of CO 2? § Balanced equation for the reaction Copyright © 2018 Cengage Learning. All Rights Reserved.

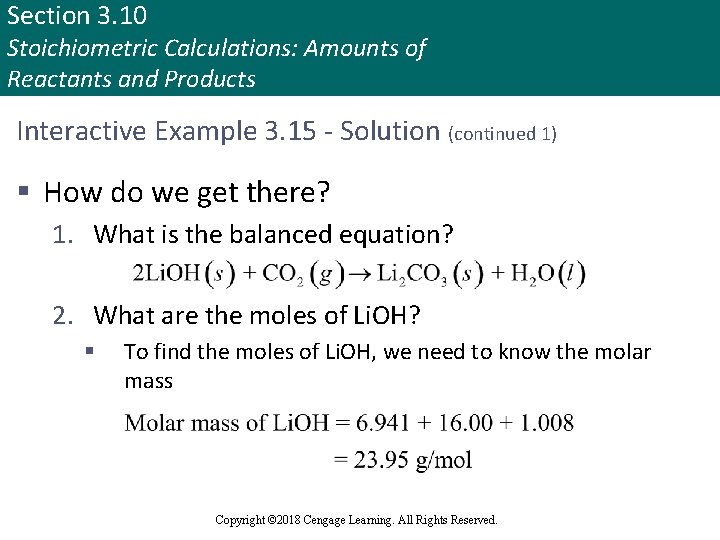
Section 3. 10 Stoichiometric Calculations: Amounts of Reactants and Products Interactive Example 3. 15 - Solution (continued 1) § How do we get there? 1. What is the balanced equation? 2. What are the moles of Li. OH? § To find the moles of Li. OH, we need to know the molar mass Copyright © 2018 Cengage Learning. All Rights Reserved.

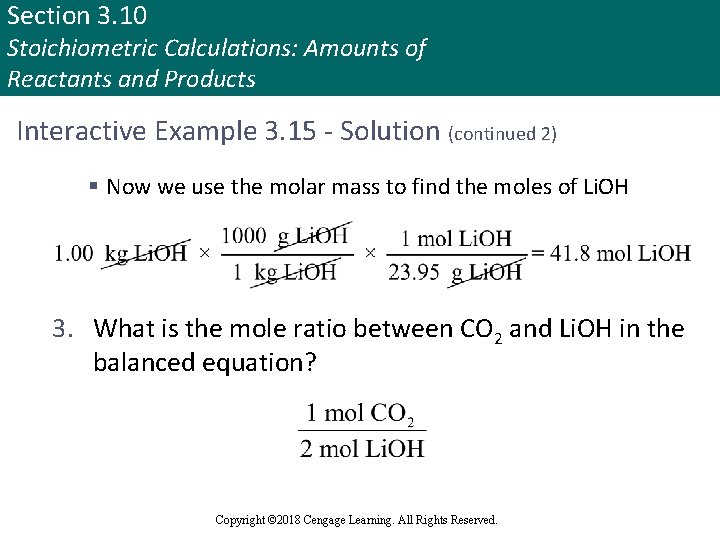
Section 3. 10 Stoichiometric Calculations: Amounts of Reactants and Products Interactive Example 3. 15 - Solution (continued 2) § Now we use the molar mass to find the moles of Li. OH 3. What is the mole ratio between CO 2 and Li. OH in the balanced equation? Copyright © 2018 Cengage Learning. All Rights Reserved.

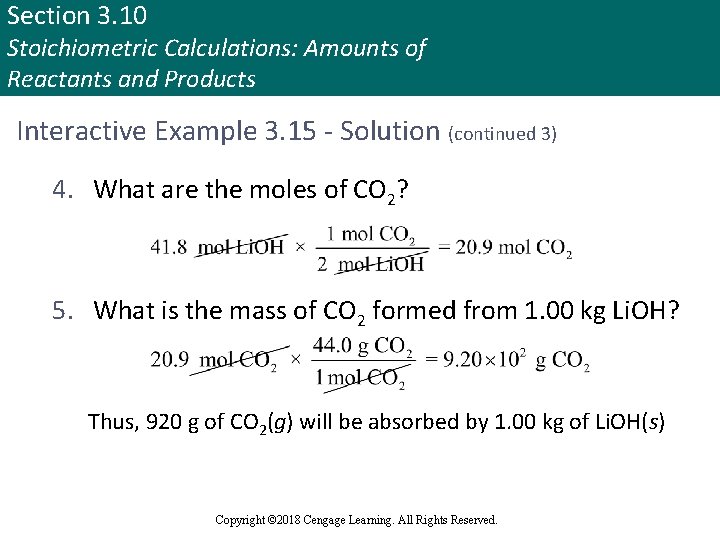
Section 3. 10 Stoichiometric Calculations: Amounts of Reactants and Products Interactive Example 3. 15 - Solution (continued 3) 4. What are the moles of CO 2? 5. What is the mass of CO 2 formed from 1. 00 kg Li. OH? Thus, 920 g of CO 2(g) will be absorbed by 1. 00 kg of Li. OH(s) Copyright © 2018 Cengage Learning. All Rights Reserved.

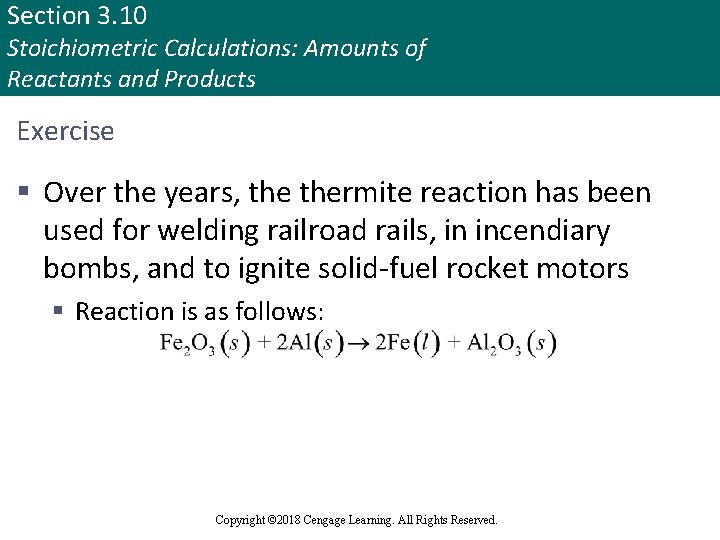
Section 3. 10 Stoichiometric Calculations: Amounts of Reactants and Products Exercise § Over the years, thermite reaction has been used for welding railroad rails, in incendiary bombs, and to ignite solid-fuel rocket motors § Reaction is as follows: Copyright © Cengage Learning. All rights reserved Copyright © 2018 Cengage Learning. All Rights Reserved. 122

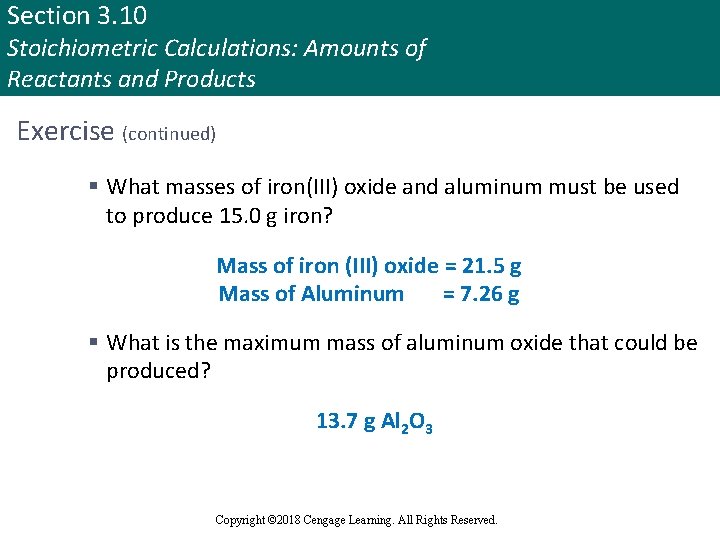
Section 3. 10 Stoichiometric Calculations: Amounts of Reactants and Products Exercise (continued) § What masses of iron(III) oxide and aluminum must be used to produce 15. 0 g iron? Mass of iron (III) oxide = 21. 5 g Mass of Aluminum = 7. 26 g § What is the maximum mass of aluminum oxide that could be produced? 13. 7 g Al 2 O 3 Copyright © Cengage Learning. All rights reserved Copyright © 2018 Cengage Learning. All Rights Reserved. 123

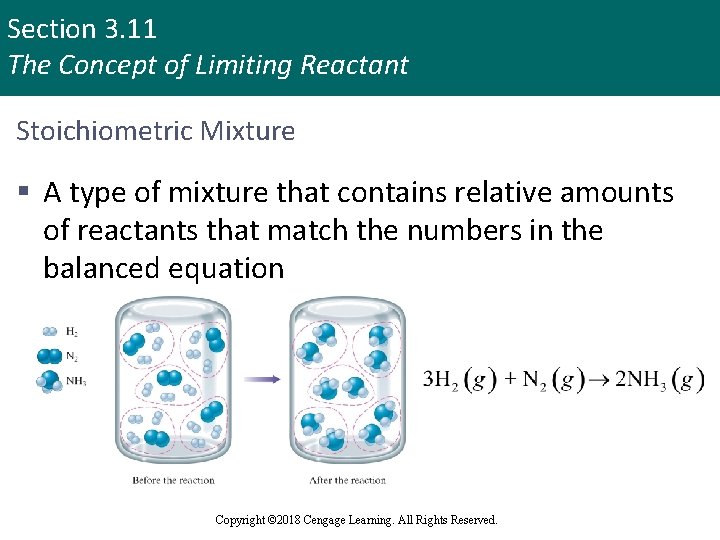
Section 3. 11 The Concept of Limiting Reactant Stoichiometric Mixture § A type of mixture that contains relative amounts of reactants that match the numbers in the balanced equation Copyright © Cengage Learning. All rights reserved Copyright © 2018 Cengage Learning. All Rights Reserved. 124

Chapter 3 Stoichiometry Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 11 The Concept of Limiting Reactant § One that runs out first and thus limits the amount of product that can form § To determine how much product can be formed from a given mixture of reactants, one must look for the reactant that is limiting § Some mixtures can be stoichiometric § All reactants run out at the same time § Requires determining which reactant is limiting Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 11 The Concept of Limiting Reactant Determination of the Limiting Reactant Using Reactant Quantities § Compare the moles of reactants to ascertain which runs out first § Use moles of molecules instead of individual molecules § Use the balanced equation to determine the limiting reactant § Determine the amount of limiting reactant formed § Use the amount of limiting reactant formed to compute the quantity of the product Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 11 The Concept of Limiting Reactant Determination of Limiting Reactant Using Quantities of Products Formed § Consider the amounts of products that can be formed by completely consuming each reactant § Reactant that produces the smallest amount of product must run out first and thus be the limiting reactant Copyright © 2018 Cengage Learning. All Rights Reserved.

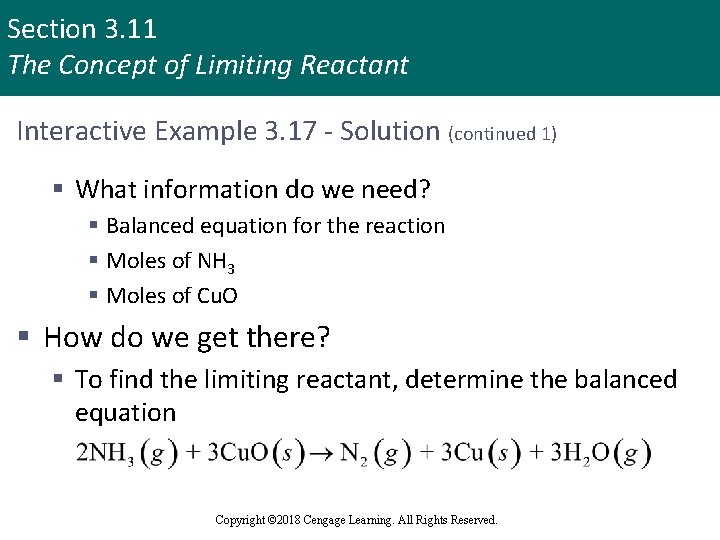
Section 3. 11 The Concept of Limiting Reactant Interactive Example 3. 17 - Stoichiometry: Limiting Reactant § Nitrogen gas can be prepared by passing gaseous ammonia over solid copper(II) oxide at high temperatures § Other products of the reaction are solid copper and water vapor § If a sample containing 18. 1 g of NH 3 is reacted with 90. 4 g of Cu. O, which is the limiting reactant? § How many grams of N 2 will be formed? Copyright © 2018 Cengage Learning. All Rights Reserved.

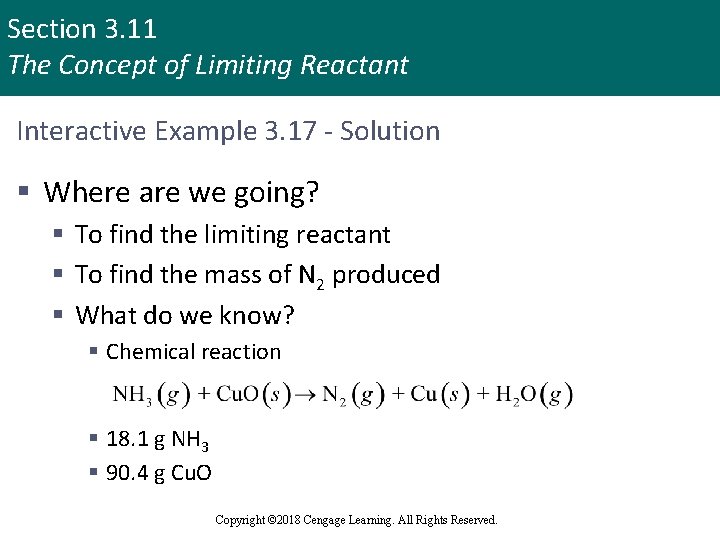
Section 3. 11 The Concept of Limiting Reactant Interactive Example 3. 17 - Solution § Where are we going? § To find the limiting reactant § To find the mass of N 2 produced § What do we know? § Chemical reaction § 18. 1 g NH 3 § 90. 4 g Cu. O Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 11 The Concept of Limiting Reactant Interactive Example 3. 17 - Solution (continued 1) § What information do we need? § Balanced equation for the reaction § Moles of NH 3 § Moles of Cu. O § How do we get there? § To find the limiting reactant, determine the balanced equation Copyright © 2018 Cengage Learning. All Rights Reserved.

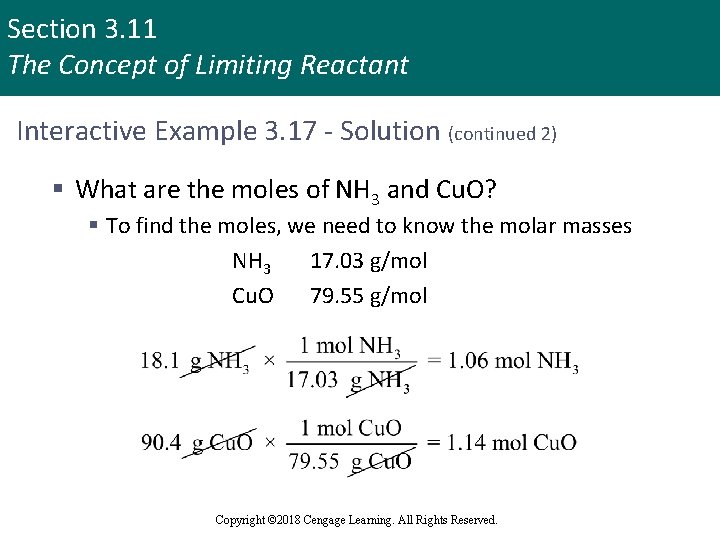
Section 3. 11 The Concept of Limiting Reactant Interactive Example 3. 17 - Solution (continued 2) § What are the moles of NH 3 and Cu. O? § To find the moles, we need to know the molar masses NH 3 17. 03 g/mol Cu. O 79. 55 g/mol Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 11 The Concept of Limiting Reactant Interactive Example 3. 17 - Solution (continued 3) A. First we will determine the limiting reactant by comparing the moles of reactants to see which one is consumed first § What is the mole ratio between NH 3 and Cu. O in the balanced equation? Copyright © 2018 Cengage Learning. All Rights Reserved.

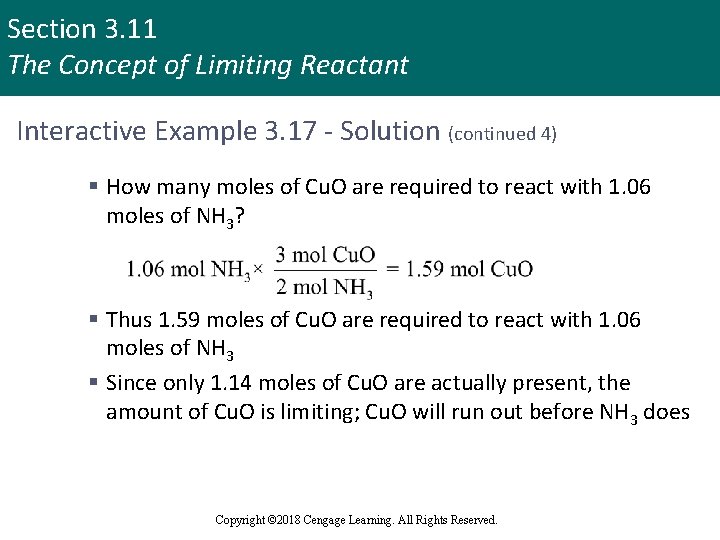
Section 3. 11 The Concept of Limiting Reactant Interactive Example 3. 17 - Solution (continued 4) § How many moles of Cu. O are required to react with 1. 06 moles of NH 3? § Thus 1. 59 moles of Cu. O are required to react with 1. 06 moles of NH 3 § Since only 1. 14 moles of Cu. O are actually present, the amount of Cu. O is limiting; Cu. O will run out before NH 3 does Copyright © 2018 Cengage Learning. All Rights Reserved.

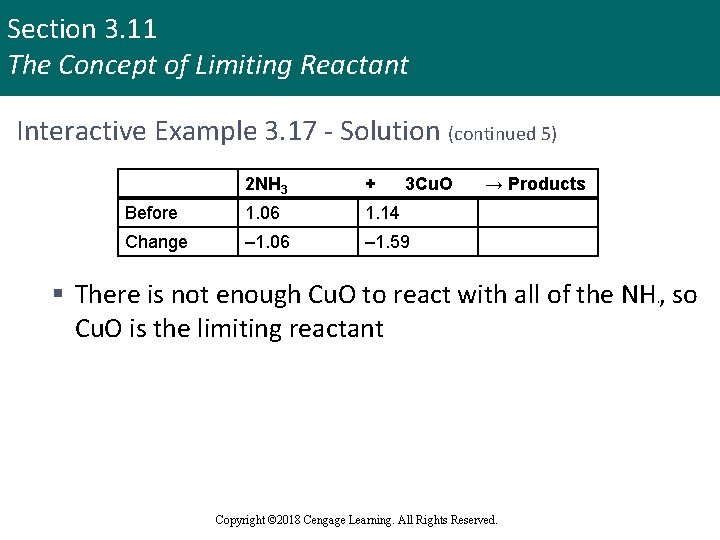
Section 3. 11 The Concept of Limiting Reactant Interactive Example 3. 17 - Solution (continued 5) 2 NH 3 + 3 Cu. O Before 1. 06 1. 14 Change – 1. 06 – 1. 59 → Products § There is not enough Cu. O to react with all of the NH , so Cu. O is the limiting reactant 3 Copyright © 2018 Cengage Learning. All Rights Reserved.

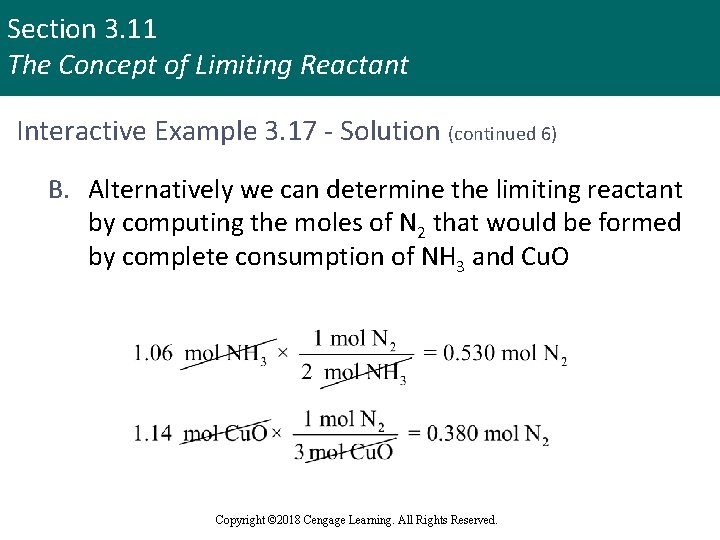
Section 3. 11 The Concept of Limiting Reactant Interactive Example 3. 17 - Solution (continued 6) B. Alternatively we can determine the limiting reactant by computing the moles of N 2 that would be formed by complete consumption of NH 3 and Cu. O Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 11 The Concept of Limiting Reactant Interactive Example 3. 17 - Solution (continued 7) § As before, we see that the Cu. O is limiting since it produces the smaller amount of N 2 § To find the mass of N 2 produced, determine the moles of N 2 formed § Because Cu. O is the limiting reactant, we must use the amount of Cu. O to calculate the amount of N 2 formed Copyright © 2018 Cengage Learning. All Rights Reserved.

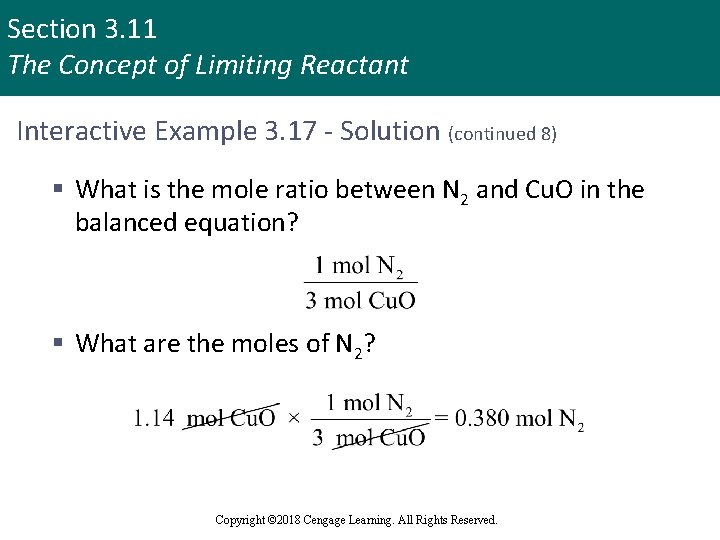
Section 3. 11 The Concept of Limiting Reactant Interactive Example 3. 17 - Solution (continued 8) § What is the mole ratio between N 2 and Cu. O in the balanced equation? § What are the moles of N 2? Copyright © 2018 Cengage Learning. All Rights Reserved.

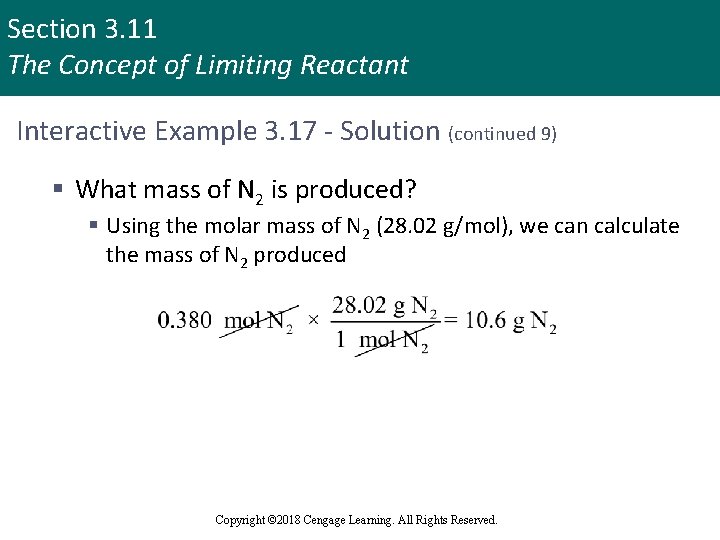
Section 3. 11 The Concept of Limiting Reactant Interactive Example 3. 17 - Solution (continued 9) § What mass of N 2 is produced? § Using the molar mass of N 2 (28. 02 g/mol), we can calculate the mass of N 2 produced Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 11 The Concept of Limiting Reactant Concept of Yield § Theoretical yield: Amount of product formed when the limiting reactant is entirely consumed § Amount of product predicted is rarely obtained because of side reactions and other complications § Percent yield: Actual yield of product § Often provided as a percentage of theoretical yield Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 11 The Concept of Limiting Reactant Interactive Example 3. 18 - Calculating Percent Yield § Methanol (CH 3 OH), also called methyl alcohol, is the simplest alcohol § It is used as a fuel in race cars and is a potential replacement for gasoline § Methanol can be manufactured by combining gaseous carbon monoxide and hydrogen Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 11 The Concept of Limiting Reactant Interactive Example 3. 18 - Calculating Percent Yield (continued) § Suppose 68. 5 kg CO(g) is reacted with 8. 60 kg H 2(g) § Calculate theoretical yield of methanol § If 3. 57 × 104 g CH 3 OH is actually produced, what is the percent yield of methanol? Copyright © 2018 Cengage Learning. All Rights Reserved.

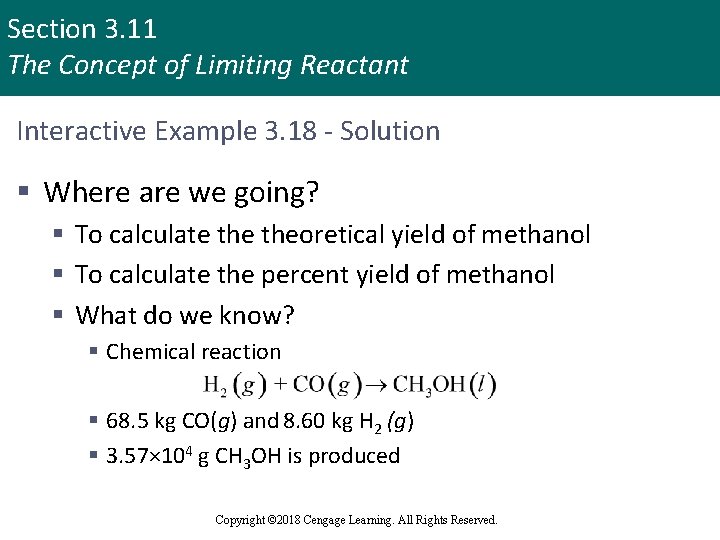
Section 3. 11 The Concept of Limiting Reactant Interactive Example 3. 18 - Solution § Where are we going? § To calculate theoretical yield of methanol § To calculate the percent yield of methanol § What do we know? § Chemical reaction § 68. 5 kg CO(g) and 8. 60 kg H 2 (g) § 3. 57× 104 g CH 3 OH is produced Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 11 The Concept of Limiting Reactant Interactive Example 3. 18 - Solution (continued 1) § What information do we need? § Balanced equation for the reaction § Moles of H 2 § Moles of CO § Which reactant is limiting § Amount of CH 3 OH produced Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 11 The Concept of Limiting Reactant Interactive Example 3. 18 - Solution (continued 2) § How do we get there? § To find the limiting reactant, balance the chemical equation § What are the moles of H 2 and CO? Copyright © 2018 Cengage Learning. All Rights Reserved.

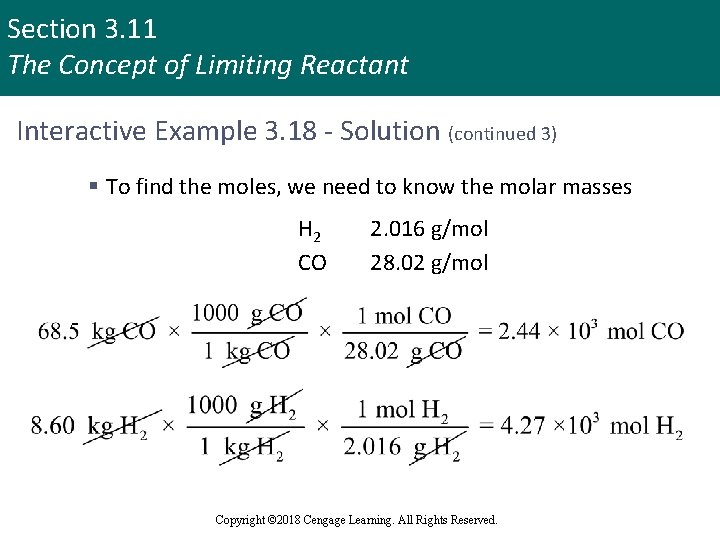
Section 3. 11 The Concept of Limiting Reactant Interactive Example 3. 18 - Solution (continued 3) § To find the moles, we need to know the molar masses H 2 CO 2. 016 g/mol 28. 02 g/mol Copyright © 2018 Cengage Learning. All Rights Reserved.

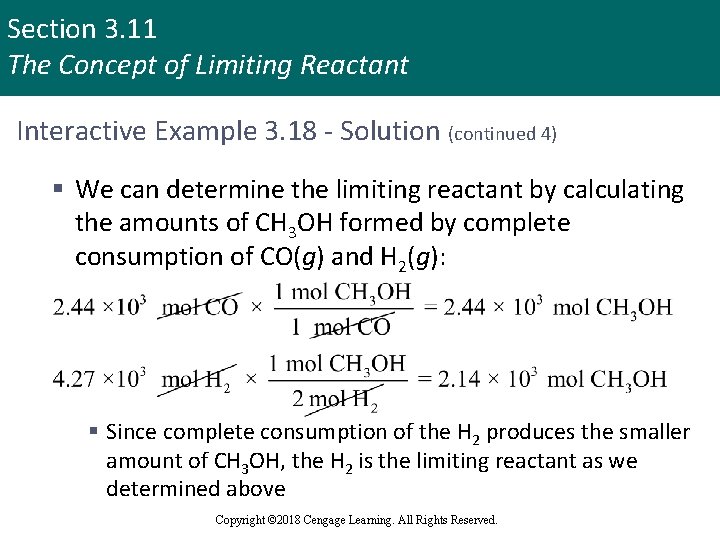
Section 3. 11 The Concept of Limiting Reactant Interactive Example 3. 18 - Solution (continued 4) § We can determine the limiting reactant by calculating the amounts of CH 3 OH formed by complete consumption of CO(g) and H 2(g): § Since complete consumption of the H 2 produces the smaller amount of CH 3 OH, the H 2 is the limiting reactant as we determined above Copyright © 2018 Cengage Learning. All Rights Reserved.

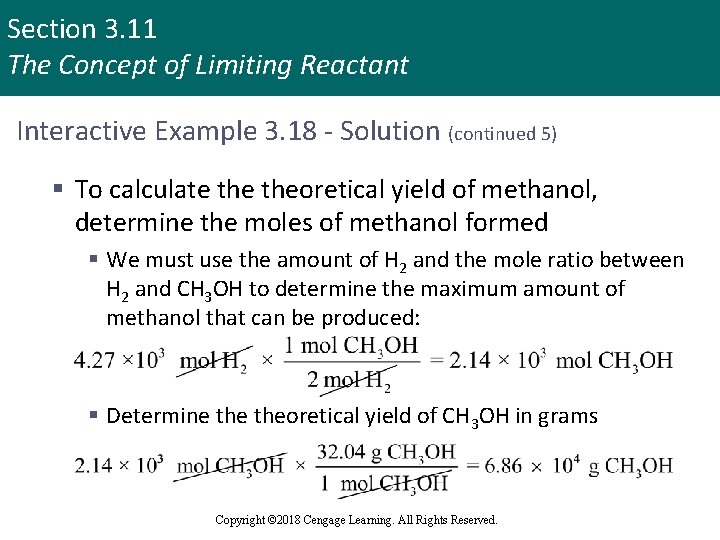
Section 3. 11 The Concept of Limiting Reactant Interactive Example 3. 18 - Solution (continued 5) § To calculate theoretical yield of methanol, determine the moles of methanol formed § We must use the amount of H 2 and the mole ratio between H 2 and CH 3 OH to determine the maximum amount of methanol that can be produced: § Determine theoretical yield of CH 3 OH in grams Copyright © 2018 Cengage Learning. All Rights Reserved.

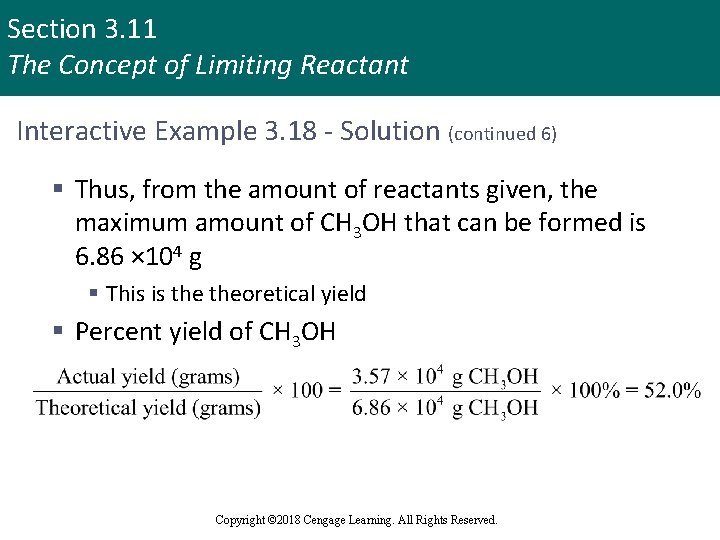
Section 3. 11 The Concept of Limiting Reactant Interactive Example 3. 18 - Solution (continued 6) § Thus, from the amount of reactants given, the maximum amount of CH 3 OH that can be formed is 6. 86 × 104 g § This is theoretical yield § Percent yield of CH 3 OH Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 11 The Concept of Limiting Reactant Problem-Solving Strategy § Solving a stoichiometry problem involving masses of reactants and products 1. Write and balance the equation for the reaction 2. Convert the known masses of substances to moles 3. Determine which reactant is limiting Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 11 The Concept of Limiting Reactant Problem-Solving Strategy (continued) 4. Using the amount of the limiting reactant and the appropriate mole ratios, compute the number of moles of the desired product 5. Convert from moles to grams, using the molar mass Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 11 The Concept of Limiting Reactant Join In (14) § In the reaction 2 A + 3 B C, 4. 0 moles of A react with 4. 0 moles of B § Which of the following choices best answers the question, “which reactant is limiting? ” Copyright © 2018 Cengage Learning. All Rights Reserved.

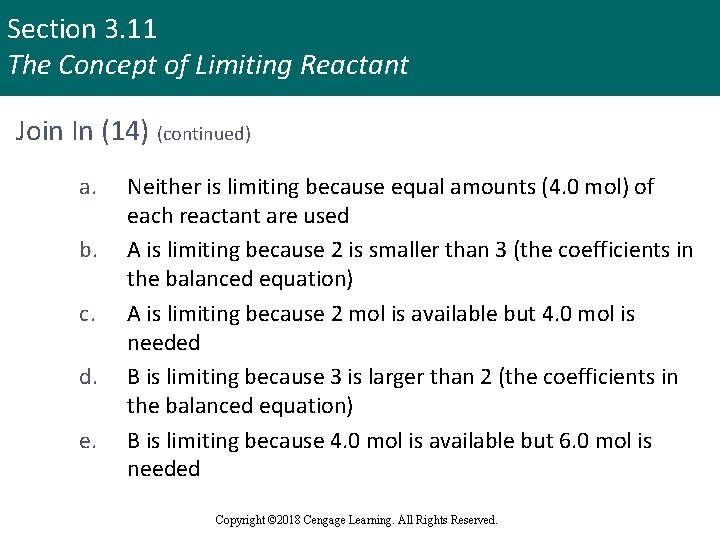
Section 3. 11 The Concept of Limiting Reactant Join In (14) (continued) a. b. c. d. e. Neither is limiting because equal amounts (4. 0 mol) of each reactant are used A is limiting because 2 is smaller than 3 (the coefficients in the balanced equation) A is limiting because 2 mol is available but 4. 0 mol is needed B is limiting because 3 is larger than 2 (the coefficients in the balanced equation) B is limiting because 4. 0 mol is available but 6. 0 mol is needed Copyright © 2018 Cengage Learning. All Rights Reserved.

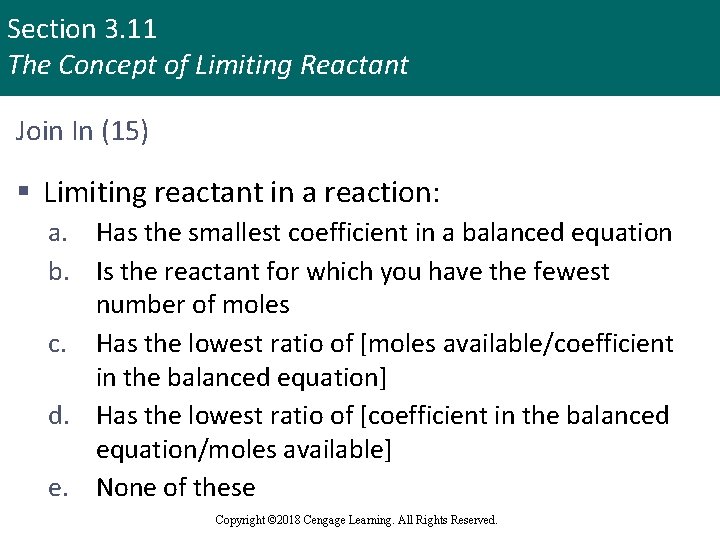
Section 3. 11 The Concept of Limiting Reactant Join In (15) § Limiting reactant in a reaction: a. Has the smallest coefficient in a balanced equation b. Is the reactant for which you have the fewest number of moles c. Has the lowest ratio of [moles available/coefficient in the balanced equation] d. Has the lowest ratio of [coefficient in the balanced equation/moles available] e. None of these Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 11 The Concept of Limiting Reactant Join In (16) § Consider the following balanced equation: A + 5 B 3 C + 4 D § Which of the following choices best answers the question, “when equal masses of A and B are reacted, which is limiting? ” Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 11 The Concept of Limiting Reactant Join In (16) (continued) a. b. c. d. If the molar mass of A is greater than the molar mass of B, then A must be limiting If the molar mass of A is less than the molar mass of B, then A must be limiting If the molar mass of A is greater than the molar mass of B, then B must be limiting If the molar mass of A is less than the molar mass of B, then B must be limiting Copyright © 2018 Cengage Learning. All Rights Reserved.

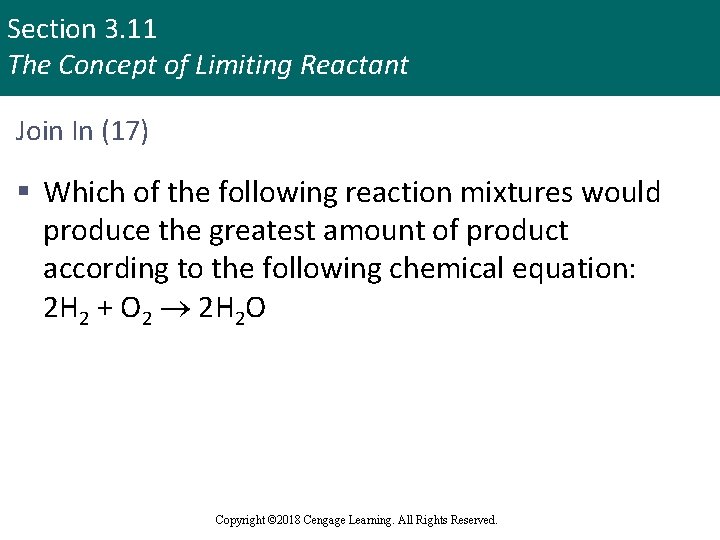
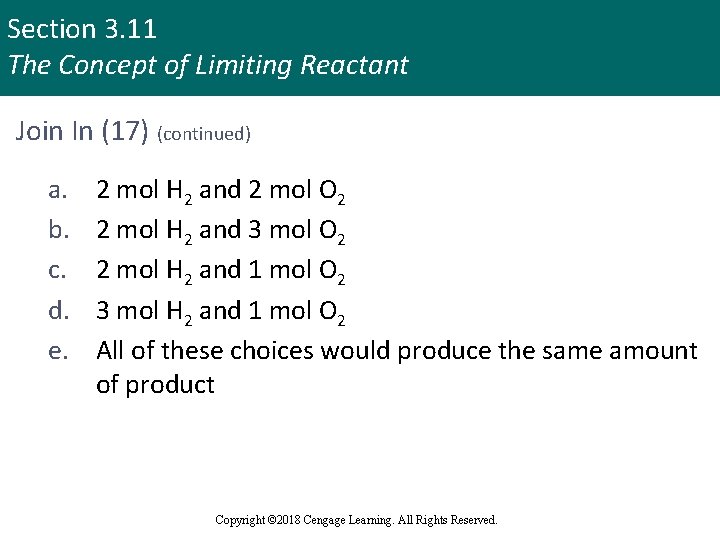
Section 3. 11 The Concept of Limiting Reactant Join In (17) § Which of the following reaction mixtures would produce the greatest amount of product according to the following chemical equation: 2 H 2 + O 2 2 H 2 O Copyright © 2018 Cengage Learning. All Rights Reserved.

Section 3. 11 The Concept of Limiting Reactant Join In (17) (continued) a. b. c. d. e. 2 mol H 2 and 2 mol O 2 2 mol H 2 and 3 mol O 2 2 mol H 2 and 1 mol O 2 3 mol H 2 and 1 mol O 2 All of these choices would produce the same amount of product Copyright © 2018 Cengage Learning. All Rights Reserved.