Chapter 3 Stoichiometry and Chemical Equations Atomic Masses







































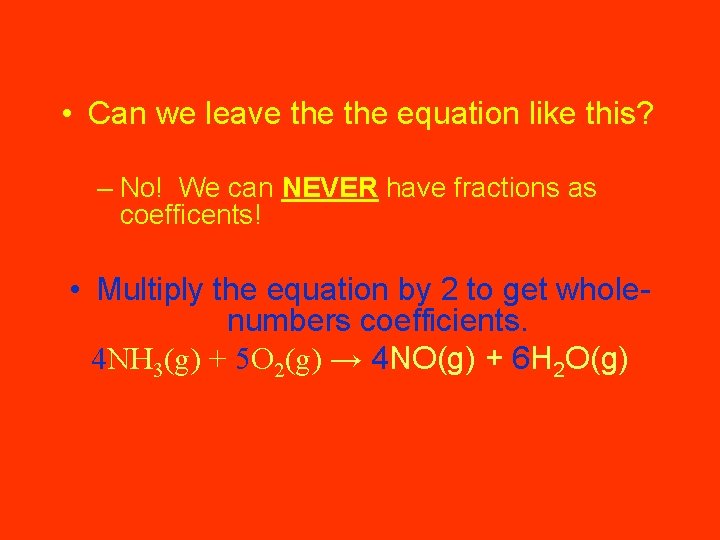
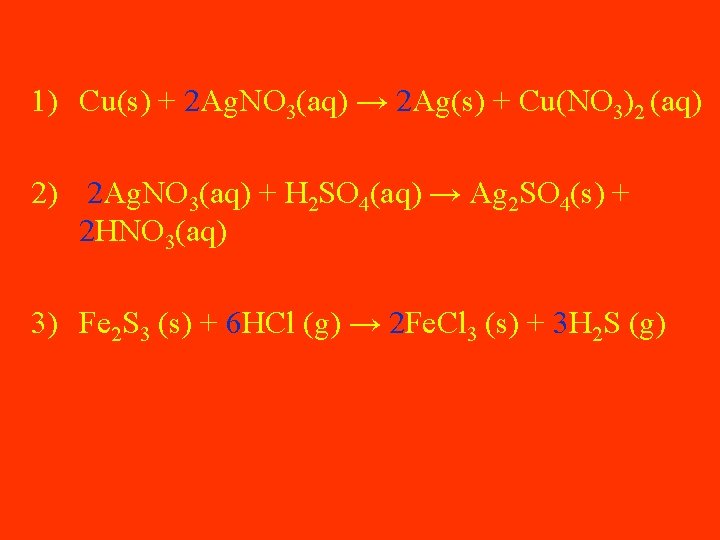














- Slides: 62

Chapter 3 Stoichiometry and Chemical Equations

Atomic Masses • Based on Carbon- 12 as a standard • Carbon- 12 is assigned 12 atomic mass units • Masses can be found using a Mass Spectrometer – This uses a high speed beam of electrons and a magnetic field to deflect ions based on there mass – The amount of deflection of the ion is compared to that of Carbon- 12 to find the mass

• The Periodic Table of Elements gives the atomic weight of each element • This number is an average of all of the isotopes which we will call the average atomic mass • To find the average atomic mass from percent of each isotope: – Change the percents to decimals – Multiply the decimals by the mass of the isotopes – Add the masses together

Solve the following: • An unknown element X has three isotopes and is found to be 84. 2% 282 X, 10. 4% 285 X and 5. 4% 289 X. What is the average atomic mass of element X? – Divide each of the percentages by 100. – 84. 2/100 = 0. 842 10. 4/100 = 0. 104 – 5. 4/100 = 0. 054

– Multiply the percentages by the mass of each isotope. • 0. 842 x 282 amu = 237. 444 amu • 0. 104 x 285 amu = 29. 64 amu • 0. 054 x 289 amu = 15. 606 amu – Add the masses together. • • 237. 444 amu + 29. 64 amu + 15. 606 amu = 282. 69 amu The average mass of element X is 282. 69 amu Is this answer correct? Yes! You are do not watch significant figures here because we are taking an average. Therefore we need to keep the whole answer. This is one of the ONLY times you should ignore significant figures.

• The average atomic mass also allows us to use something know as “counting by weighing” • Solve the following: You now know that the average atomic mass of element X is 282. 69 amu. If you are told to give me a sample of 725 atoms of element X, what would be the quickest way to do it?

• While you could count out 725 atoms, it is not time efficent or possible when we are talking about atoms, therefore we will count by weighing them: – 725 atoms X │ 282. 69 amu = 204950 amu │ 1 atom X • Is this the correct answer? • No! Watch significant figures! • The correct answer is 2. 05 x 105 amu

The Mole • Defined as the number of carbon atoms in exactly 12 grams of 12 C. • Also known as Avogadro’s number. • One mole = 6. 022 x 1023 units of that substance. • One mole of atoms is also defined as a sample of the element equal to the element’s atomic mass expressed in grams.

Solve the following: • If you have 537 mg of sulfur, how many sulfur atoms do you have?

• First you must convert from milligrams to grams. – There are 1000 mg in 1 gram. • Second you must convert from grams to moles. – There are 32. 07 grams of sulfur in 1 mole. • Lastly you must go from moles to atoms. – There are 6. 02 x 1023 atoms in a mole. 537 mg │ 1 mole │ 6. 02 x 1023 atoms │1000 mg│ 32. 07 g│ 1 mole Answer: 1. 01 x 1022 atoms

Gram Formula Mass • Also known as the molar mass. This amount is the mass of one mole of any element or compound. • To find the molar mass: – Separate the formula into the elements. – Identify how many atoms of each element there is. – Look up the mass of each element. – Multiply the masses by the number of atoms present for that element.

Solve the following: • Please find the gram formula mass for each of the following: a) b) c) d) e) H 2 O H 2 SO 4 (NH 4)3 PO 4 Ferric Carbonate Chloric Acid

a) 2 H and 1 O – 2(1. 0079) + 1(15. 9994) = 18. 015 g b) 2 H’s, 1 S, 4 O’s • 2(1. 0079) + 1(32. 07) + 4(15. 9994) = 98. 08 g c) 3 N’s, 12 H’s, 1 P, 4 O’s • 3(14. 01) + 12(1. 0079) + 1(30. 97) + 4(15. 9994) = 149. 09 g d) Fe 2(CO 3)3 = 2 Fe’s, 3 C’s, 9 O’s • 2(55. 85) + 3(12. 01) + 9(15. 9994) = 291. 72 g e) HCl. O 3 = 1 H, 1 Cl, 3 O’s • 1(1. 0079) + 1(35. 453) + 3(15. 9994) = 84. 46 g • For gram formula masses you should keep a few decimal places. These numbers are not measurements so you should not round too soon.

Solve the following: • Please calculate the number of atoms of Hydrogen there are present in 250 grams of Ammonium Sulfate.

• First we must go from grams to moles using the gram formula mass. – There are 132. 15 grams in 1 mole of (NH 4)2 SO 4 • Next we must go from moles to molecules. – There are 6. 02 x 1023 molecules in 1 mole • Lastly we must go from molecules to atoms. – There are 8 H atoms in every 1 molecule (NH 4)2 SO 4 250 g│1 mole │6. 02 x 1023 molecules │ 8 H atoms │132. 15 g│ 1 mole (NH 4)2 SO 4 │1 molecule Answer: 9. 1 x 1024 Hydrogen atoms

Percent Composition • While the chemical formula of a compound describes the compound the number of atoms it contains, we can also describe compounds by the percentage of mass of each element present. • This may be referred to as percent mass or percent weight.

To find the Percent Composition: • Find the gram formula mass for the compound. • Then find the mass of each of the elements in the compound. – REMEMBER – You must multiply the mass of the element by the number of atoms of that element present in the compound. • Divide the mass of each of the elements in the compound by the gram formula mass. • Multiply the decimals by 100.

Solve the following: • What is the percent composition of Stannic Phosphate?

• First write the formula for the compound. – Sn 3(PO 4)4 • Next find the gram formula mass. – 3(118. 7) + 4(30. 97) + 16(15. 9994) = 735. 97 g • Then find the mass of each of the elements present in the compound. – Sn = 356. 1 g P = 123. 88 g O = 255. 99 g • Lastly divide each of the element masses by the gram formula mass and multiply by 100. Sn = 356. 1 g x 100 735. 97 g Sn = 48. 4% P = 123. 88 g x 100 735. 97 g P = 16. 8 % O = 255. 99 g x 100 735. 97 g O = 34. 8 %

Determining Formulas • Empirical Formula- a formula for a compound where the subscripts are as reduced as possible. • Molecular Formula- a formula for a compound that fits a specific molecule and does not have to be reduced.

Determining the Empirical Formula 1) Start with the percent composition (if you do not have the percent composition you must first find it). 2) Change the percents to decimals. 3) Assume a 100 grams sample and multiply by the decimals by 100. 4) Divide the masses by the mass of the element. 5) Divide each of the numbers by the smallest number found in step 4. 6) Take the numbers found in step 5 and insert them into the chemical formula.

Solve the following: • What is the empirical formula of a compound that is 25 % Hydrogen and 75 % Carbon? • Hydrogen Carbon • 25/100 = 0. 25 75/100 = 0. 75 • 0. 25 x 100 g = 25 g 0. 75 x 100 g = 75 g • 25 g / 1. 0079 g = 25 H 75 g/ 12. 01 g = 6. 25 C • 25 H/ 6. 25 = 4 H 6. 25 C/ 6. 25 = 1 C • The empirical formula is CH 4

Solve the following: • What is the empirical formula for a compound that is 58 grams and contains Carbon and 10 grams of Hydrogen?

• First we need to find the percent composition. – (10 g H/ 58 g)x 100 = 17. 24% H – (48 g C/ 58 g)x 100 = 82. 76% C • Next we need to convert from percents to grams. – (17. 24% / 100) x 100 g = 17. 24 g H – (82. 76% / 100) x 100 g = 82. 76 g C • Then we need to find the number of atoms of each element in the formula. – 17. 24 g H/ 1. 0079 g = 17. 1 H – 82. 76 g C/ 12. 01 g = 6. 89 C

• Divide each number by the smallest number. – 17. 1 H/ 6. 89 = 2. 48 or 2. 5 H – 6. 89 C/ 6. 89 = 1. 00 H • Place the numbers into the chemical formula. – C 1 H 2. 5 • What is wrong with this formula? – You cannot have decimal subscripts. (There cannot be half of a Hydrogen atom) – C 2 H 5 (Multiply the formula by 2 to get whole numbers)

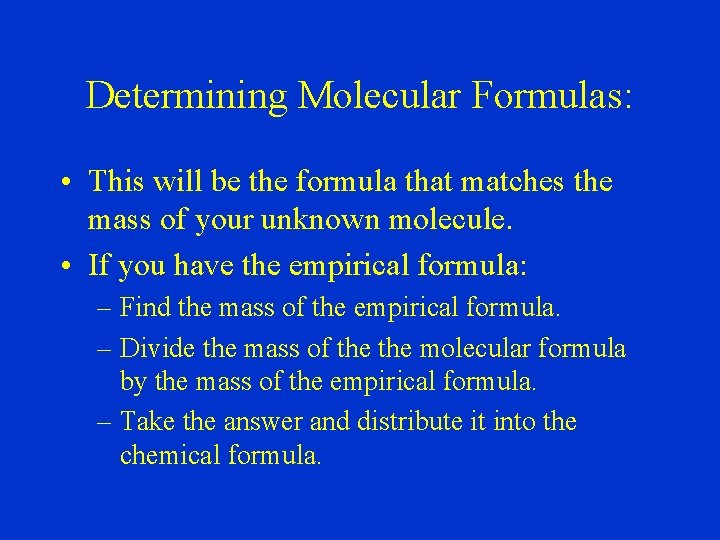
Determining Molecular Formulas: • This will be the formula that matches the mass of your unknown molecule. • If you have the empirical formula: – Find the mass of the empirical formula. – Divide the mass of the molecular formula by the mass of the empirical formula. – Take the answer and distribute it into the chemical formula.

Solve the following: • What is the molecular formula of a compound that is 58 grams and contains Carbon and 10 grams of Hydrogen?

• We already found the empirical formula for the compound to be C 2 H 5. • Find the mass of the empirical formula. – 2(12. 01 g) + 5(1. 0079 g) = 29. 06 g • Divide the molecular mass by the empirical mass. – 58 g / 29. 06 g = 1. 995 or 2 • Take the answer and distribute it into the formula. – (C 2 H 5)2 = C 4 H 10

Solve the following: • Analysis of aspirin shows it is 60. 0% C, 4. 48% H and 35. 5% O. If the molecular mass of this substance is 180. 2 -u, what is its molecular formula?

• First we do not need to find the empirical formula, we can go straight to the molecular formula. • How can we do this? – Instead of assuming a 100 gram sample, we will use the mass of the sample that is given to us for the molecular formula. – (60. 0 % / 100) x 180. 2 g = 108. 12 g C – (4. 48 % / 100) x 180. 2 g = 8. 073 g H – (35. 5 % / 100) x 180. 2 g = 63. 971 g O

• Next we need to divide the masses by the mass of each element. – 108. 12 g C / 12. 01 g = 9. 00 C – 8. 073 g H / 1. 0079 g = 8. 01 H – 63. 971 g O / 15. 9994 g = 4. 00 O • Place the numbers into the chemical formula. – C 9 H 8 O 4 – If we check the gram formula mass: – 9(12. 01) + 8(1. 0079) + 4(15. 9994) = 180. 15 g

Chemical Changes • A chemical change involves a reorganization of the atoms in one or more substances. • Example: Combustion of Methane (CH 4) CH 4(g) + O 2 → CO 2(g) + H 2 O(g) reactants products Notice the atoms are rearranged. Bonds have been broken and new bonds formed.

Conservation of Atoms • In an ordinary chemical reaction, atoms are neither created nor destroyed. • All atoms present in the reactants must be accounted for among the products.

Balancing Chemical Equations CH 4(g) + O 2 (g) → CO 2(g) + H 2 O(g) Equation is not balanced! CH 4(g) + 2 O 2(g) → CO 2(g) + 2 H 2 O(g) Equation is balanced!

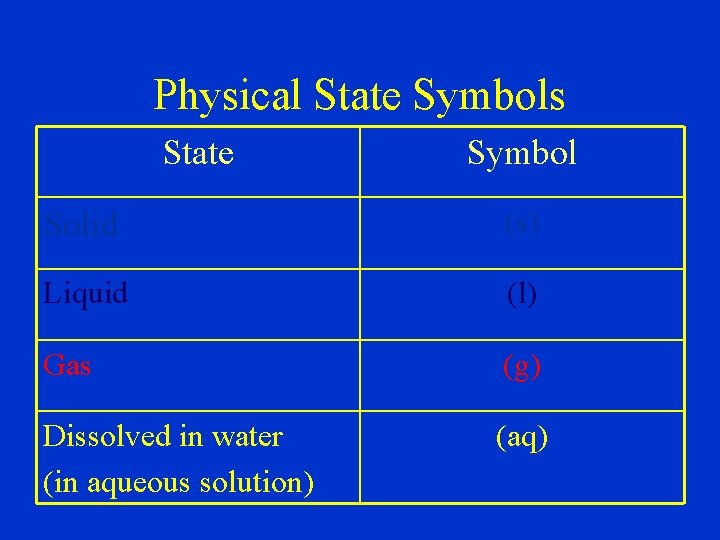
Physical State Symbols State Symbol Solid (s) Liquid (l) Gas (g) Dissolved in water (in aqueous solution) (aq)

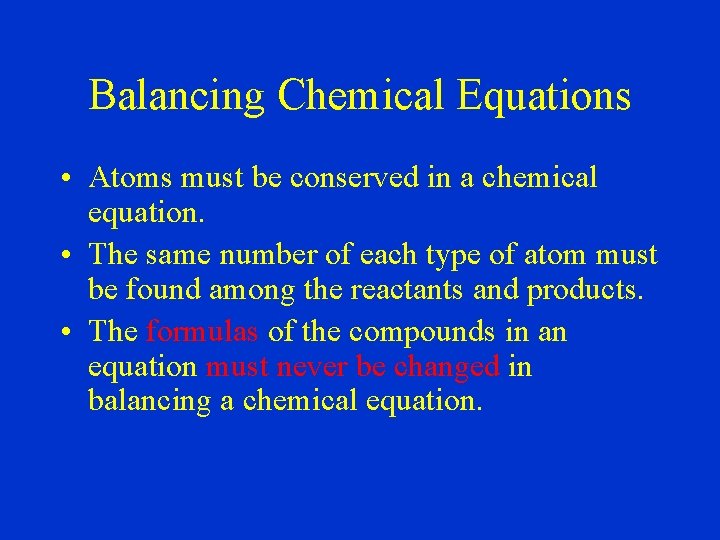
Balancing Chemical Equations • Atoms must be conserved in a chemical equation. • The same number of each type of atom must be found among the reactants and products. • The formulas of the compounds in an equation must never be changed in balancing a chemical equation.

Steps to Balancing Equations I • 1. Determine what reaction is occurring. What are the reactants, the products, and the physical states involved? • 2. Write the unbalanced equation that summarizes the reaction described in step 1. • 3. Balance the equation by inspection, starting with the most complicated molecule(s). Determine what coefficients are necessary so that the same number of each type of atom appears on both reactant and product sides.

Steps to Balancing Equations II • 1. Balance metals. • 2. Balance polyatomic ions. • 3. Balance nonmetals other than H and O. • 4. Balance H and O • DO NOT CHANGE ANY FORMULAS!

Decomposition of Ammonium Dichromate

Decomposition of Ammonium Dichromate • When ignited, solid ammonium dichromate decomposes into chromium (III) oxide, nitrogen gas, and water vapor. • The formula for the reactant is (NH 4)2 Cr 2 O 7(s). • The formula for chromium (III) oxide can be determined by recognizing the Roman numeral III means Cr 3+ ions. Since each oxide ion is 2 -, the formula is Cr 2 O 3. The unbalanced equation is: (NH 4)2 Cr 2 O 7(s)→ Cr 2 O 3(s) + N 2(g) + H 2 O(g)

(NH 4)2 Cr 2 O 7(s)→ Cr 2 O 3(s) + N 2(g) + H 2 O(g) • Note that the nitrogen and chromium are balanced but hydrogen and oxygen are not. • A coefficient of 4 for H 2 O balances the hydrogen atoms. In balancing the hydrogen atoms, the oxygen atoms are also balanced. (NH 4)2 Cr 2 O 7(s)→ Cr 2 O 3(s) + N 2(g) + 4 H 2 O(g) The equation is balanced.

Solve the following: • At 1000 o. C, ammonia gas, NH 3(g), reacts with oxygen gas to form gaseous nitric oxide, NO(g), and water vapor.

The unbalanced equation for the reaction is: NH 3(g) + O 2(g) → NO(g) + H 2 O(g) A coefficient of 2 for NH 3 and a coefficient of 3 for H 2 O give six atoms of hydrogen on both sides: 2 NH 3(g) + O 2(g) → NO(g) + 3 H 2 O(g) The nitrogen can be balanced with a coefficient of 2 for NO: 2 NH 3(g) + O 2(g) → 2 NO(g) + 3 H 2 O(g) There are 2 atoms of left and 5 atoms on the right. It can be balanced with a coefficient of 5/2 for O 2: 2 NH 3(g) + 5/2 O 2(g) → 2 NO(g) + 3 H 2 O(g)
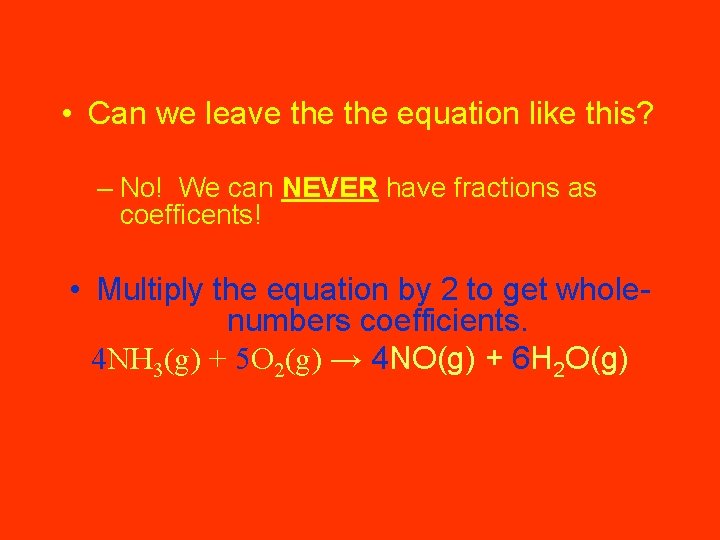
• Can we leave the equation like this? – No! We can NEVER have fractions as coefficents! • Multiply the equation by 2 to get wholenumbers coefficients. 4 NH 3(g) + 5 O 2(g) → 4 NO(g) + 6 H 2 O(g)

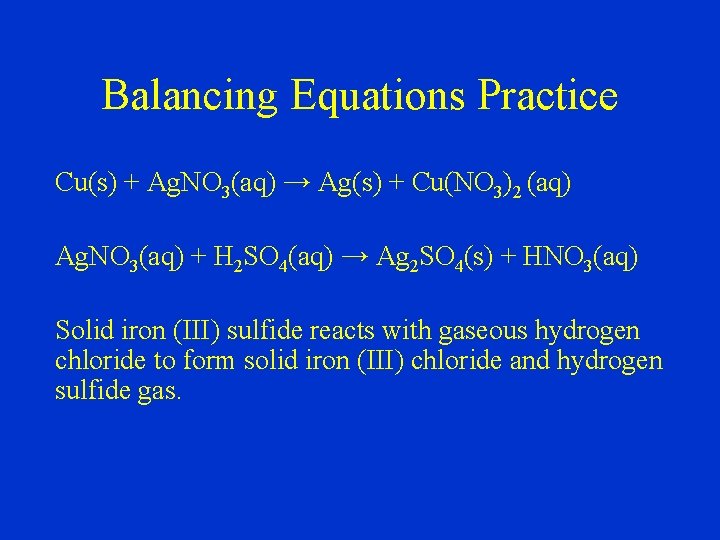
Balancing Equations Practice Cu(s) + Ag. NO 3(aq) → Ag(s) + Cu(NO 3)2 (aq) Ag. NO 3(aq) + H 2 SO 4(aq) → Ag 2 SO 4(s) + HNO 3(aq) Solid iron (III) sulfide reacts with gaseous hydrogen chloride to form solid iron (III) chloride and hydrogen sulfide gas.
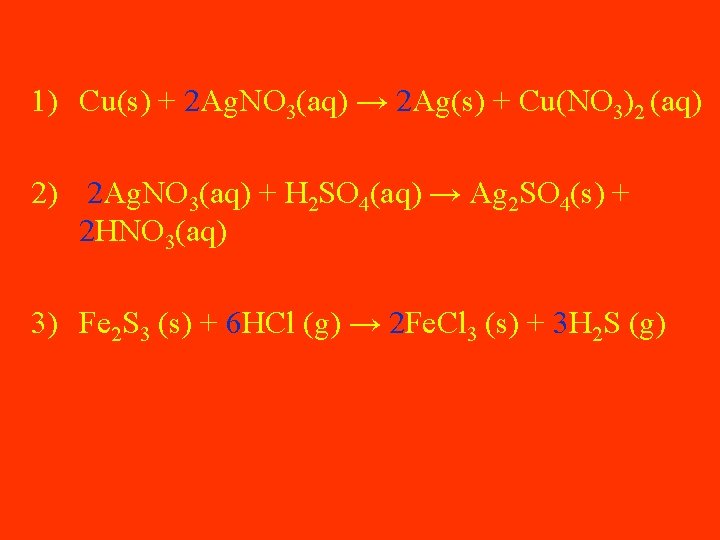
1) Cu(s) + 2 Ag. NO 3(aq) → 2 Ag(s) + Cu(NO 3)2 (aq) 2) 2 Ag. NO 3(aq) + H 2 SO 4(aq) → Ag 2 SO 4(s) + 2 HNO 3(aq) 3) Fe 2 S 3 (s) + 6 HCl (g) → 2 Fe. Cl 3 (s) + 3 H 2 S (g)

Stoichiometric Calculations: Amounts of Reactants and Products • Remember chemical equations represent numbers of molecules, not masses of molecules. • Counting is always done by weighing. • Remember, before doing any calculations involving a chemical reaction, be sure the equation for the reaction is balanced.

• Write the equation: – C 3 H 5(g) + O 2(g) → CO 2(g) + H 2 O(g) • The balanced equation: – C 3 H 5(g) + 5 O 2(g) → 3 CO 2(g) + 4 H 2 O(g) • This equation means that 1 mole of C 3 H 5 reacts with 5 moles of O 2 to produce 3 moles of CO 2 and 4 moles of H 2 O.

Solve the following: • What mass of O 2 will react with 96. 1 grams of propane (C 3 H 8)?

C 3 H 8(g) + 5 O 2(g) → 3 CO 2(g) + 4 H 2 O(g) 1. Find the number of moles of the propane. 96. 1 g C 3 H 8| 1 mol C 3 H 8| = 2. 18 mol C 3 H 8 44. 1 g C 3 H 5 2. Use the balanced equation to set up a mole ratio to calculate the number of moles of the desired reactant or product. 2. 18 mol C 3 H 8| 5 mol O 2| = 10. 9 mol O 2 1 mol C 3 H 5 3. Convert from moles to units required by the problem. 10. 9 mol O 2| 32 g O 2| = 349 g O 2 1 mole O 2

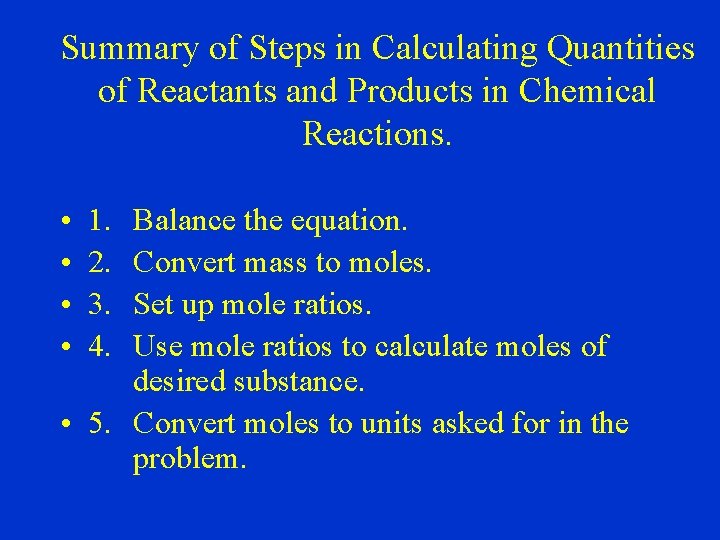
Summary of Steps in Calculating Quantities of Reactants and Products in Chemical Reactions. • • 1. 2. 3. 4. Balance the equation. Convert mass to moles. Set up mole ratios. Use mole ratios to calculate moles of desired substance. • 5. Convert moles to units asked for in the problem.

Practice Problems. 1. Solid lithium hydroxide is used in space vehicles to remove exhaled carbon dioxide from the living environment by forming solid lithium carbonate and liquid water. What mass of gaseous carbon dioxide can be absorbed by 1. 00 kg of lithium hydroxide? Answer: 920. g of CO 2

• • • First write the equation: Li. OH(s) + CO 2 (g) Li 2 CO 3 (s) + H 2 O (g) Balance the equation: 2 Li. OH(s) + CO 2 (g) Li 2 CO 3 (s) + H 2 O (g) Work out the stoichiometry: 1. 00 kg Li. OH| 1000 g | 1 mole Li. OH = 41. 757 | 1 kg | 23. 948 g Li. OH moles • 41. 757 moles Li. OH | 1 mole CO 2 | 44 g. CO 2 = | 2 mole Li. OH | 1 mole CO 2 Answer: 918. 66 grams or 919 grams CO 2

Baking soda (Na. HCO 3) is often used as an antacid. It neutralizes excess hydrochloric acid secreted by the stomach: Na. HCO 3 (s) + HCl (aq) → Na. Cl (aq) + H 2 O (l) + CO 2(aq) Milk of magnesia, which is an aqueous suspension of Mg(OH)2, is also used as an antacid: Mg(OH)2(aq) + 2 HCl(aq)→ 2 H 2 O(l) + Mg. Cl 2(aq) Which is the more effective antacid per gram, Na. HCO 3, or Mg(OH)2?

• The Milk of magnesia is a better antacid. • How do we know? – By looking at the balanced equations we can see that for every one mole of baking soda we will neutralize one mole of hydrochloric acid. – For every one mole of magnesia we will neutralize 2 moles of hydrochloric acid. – Therefore the milk of magnesia is twice as effective because it will neutralize twice as much hydrochloric acid.

Calculations Involving a Limiting Reactant • Limiting reaction problems are where the quantities of two (rarely more than two) reactants are stated. • The limiting reactant controls the amount of product that can form. • The problem allows the assumption of 100% reaction for one of the substances. • It is then necessary to determine which of the two would be used up. . . the “limiting” reactant. • The limiting reactant is then used with the “mole ratio” to compute the amount of product produced.

Solving Problems with a Limiting Reactant 1) Write the balanced equation for the reaction. 2) For each of the reactants, convert to moles of product (it must be the same product). 3) The reactant that gives you the least moles of product is the limiting reactant. 4) Use the limiting reactant to find the amount of product(s) that the problem asks for.

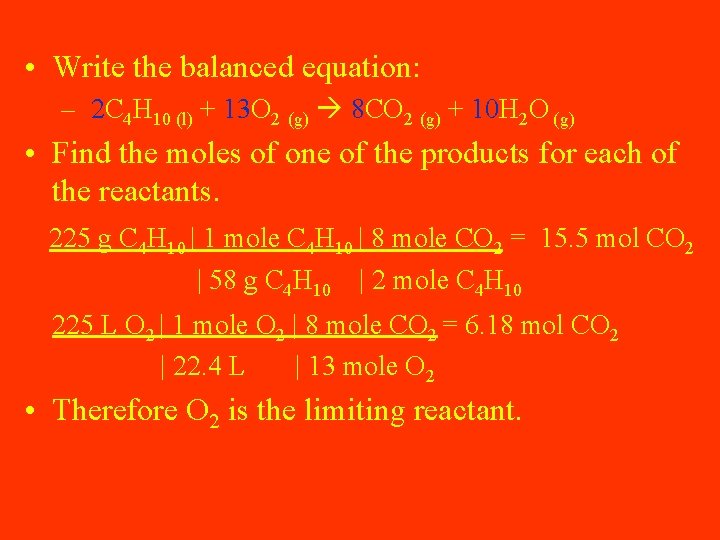
Solve the following: • If you burn 225 grams of Butane (C 4 H 10) in a container with only 225 L of O 2, how many grams of each product will be produced?

• Write the balanced equation: – 2 C 4 H 10 (l) + 13 O 2 (g) 8 CO 2 (g) + 10 H 2 O (g) • Find the moles of one of the products for each of the reactants. 225 g C 4 H 10 | 1 mole C 4 H 10 | 8 mole CO 2 = 15. 5 mol CO 2 | 58 g C 4 H 10 | 2 mole C 4 H 10 225 L O 2 | 1 mole O 2 | 8 mole CO 2 = 6. 18 mol CO 2 | 22. 4 L | 13 mole O 2 • Therefore O 2 is the limiting reactant.

• Use the limiting reactant to solve for both products. 6. 18 mol CO 2 | 44 g CO 2 = 272 g CO 2 |1 mole CO 2 225 L O 2 | 1 mole O 2 | 10 mole H 2 O | 18 g H 2 O = 139 g H 2 O | 22. 4 L O 2 | 13 mole O 2 |1 mole H 2 O

Percent Yield • This will compare the actual yield you found in an experiment (in the lab) to theoretical value that you calculated. • Actual Yield (experimental value) x 100 Theoretical Yield (calculated value)

End of Chapter!!