Chapter 3 States of Matter I Section 3




- Slides: 8

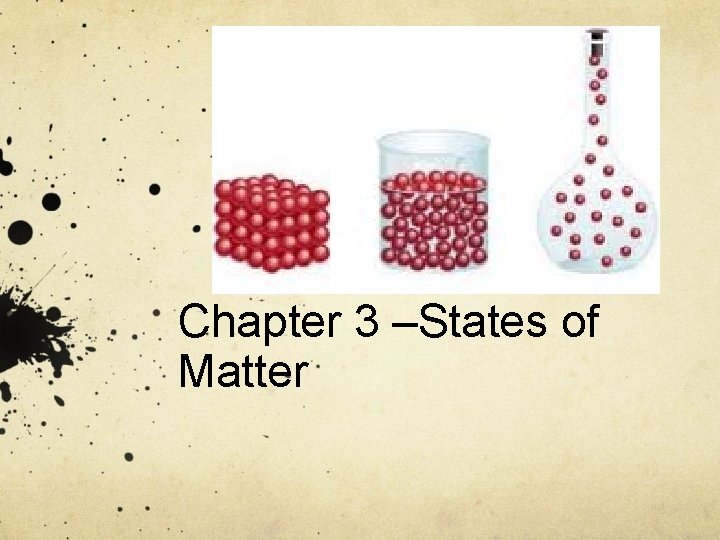
Chapter 3 –States of Matter

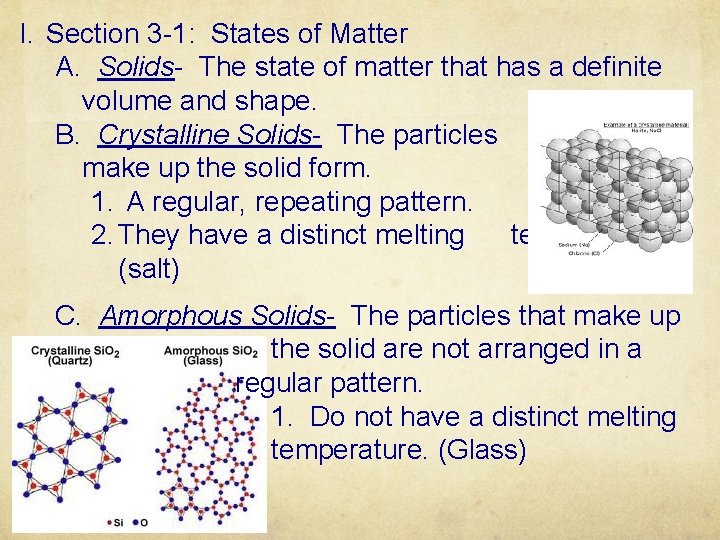
I. Section 3 -1: States of Matter A. Solids- The state of matter that has a definite volume and shape. B. Crystalline Solids- The particles that make up the solid form. 1. A regular, repeating pattern. 2. They have a distinct melting temperature. (salt) C. Amorphous Solids- The particles that make up the solid are not arranged in a regular pattern. 1. Do not have a distinct melting temperature. (Glass)

D. Liquid-The state of matter that has a definite volume but no shape of its own. E. Fluid-a substance that flows. F. Viscosity-A liquid's resistance to flow. G. Surface Tension-An inward pull among the molecules of a liquid that brings the molecules on the surface closer together.

II. Section 3 -2: Changes of State A. Melting-The change in state from a solid to a liquid. B. Melting point-The specific temperature at which a substance turns from a solid to a liquid. C. Freezing-The change from a liquid to a solid.

D. Gas-The state of matter that takes the volume and shape of its container. E. Vaporization-Particles in a liquid gain enough energy to move independently, forming a gas. F. Evaporation-Vaporization that takes place only on the surface of a liquid. G. Boiling-When a liquid changes to a gas below its surface as well as at the surface. H. Boiling Point-The temperature at which a liquid boils.

I. Condensation-The change in state from a gas to a liquid. J. Sublimation-Particles of a solid do not pass through the liquid state as they form a gas. III. Section 3 -3 Gases A. Pressure-The outward force of the gas particles on its container.

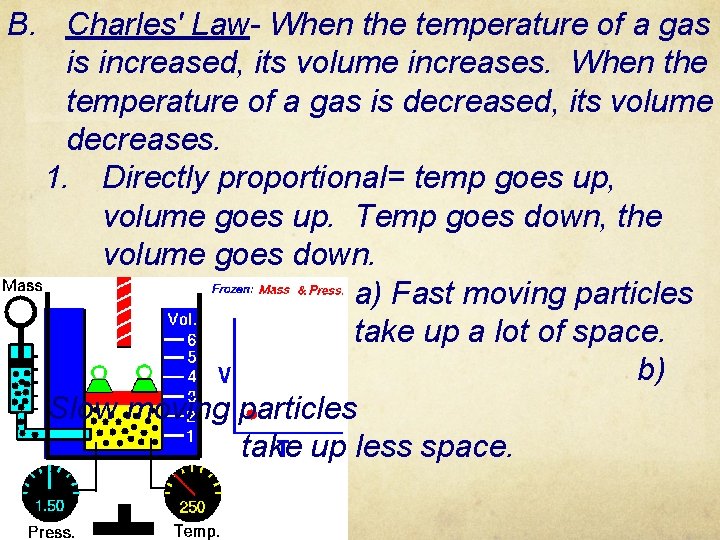
B. Charles' Law- When the temperature of a gas is increased, its volume increases. When the temperature of a gas is decreased, its volume decreases. 1. Directly proportional= temp goes up, volume goes up. Temp goes down, the volume goes down. a) Fast moving particles take up a lot of space. b) Slow moving particles take up less space.

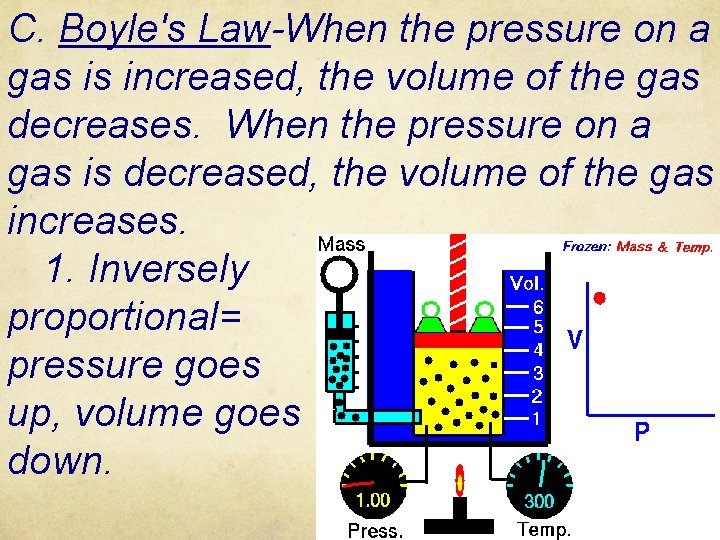
C. Boyle's Law-When the pressure on a gas is increased, the volume of the gas decreases. When the pressure on a gas is decreased, the volume of the gas increases. 1. Inversely proportional= pressure goes up, volume goes down.