Chapter 3 Section 3 Notes Phase Changes Phase







- Slides: 14

Chapter 3 Section 3 Notes Phase Changes

Phase Changes • A phase change is a reversible physical change that occurs when a substance changes from one state of matter to another. • Examples: – – – Melting Freezing Vaporization Condensation Sublimation Deposition

Energy and Phase Change • Energy is either absorbed or released during a phase change. • Endothermic change means that the change is absorbing energy from its surroundings. • Exothermic change means that the change is releasing energy to its surroundings.

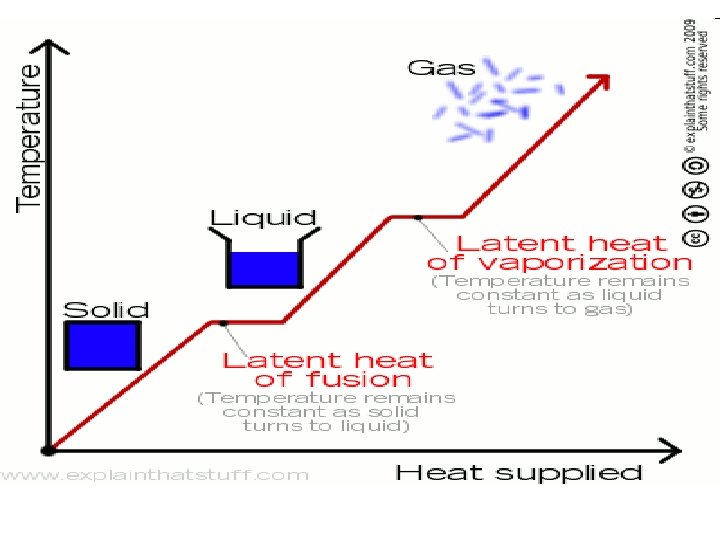
Heat of Fusion/Heat of Vaporization • The amount of energy absorbed or released during a phase change is different for different substances. • Heat of Fusion is the amount of energy required to melt one gram of a substance. • Heat of Vaporization is the amount of energy required to vaporize one gram of a substance.


Melting or Fusion • Occurs when a substance absorbs heat energy • The rigid crystal structure of the particles breaks down so that the particles are free to move around one another. • Melting Point: the temperature at which a solid changes to a liquid (physical property)

Freezing • Occurs when substance loses heat energy • Particles become rigid and locked into place • Freezing Point: the temperature at which the substance changes from liquid to solid. (physical property) • Melting point and freezing point are equal!

Vaporization • Occurs when liquid absorbs heat energy • Particles are free to move apart and expand. • Boiling Point: The temperature that a liquid changes to a gas. (physical property)

Evaporation Vs. Vaporization • Both are phase changes from liquid to gas. • Vaporization takes place all throughout the liquid. • Evaporation only takes place at the surface of the liquid

Condensation • Occurs when the gas loses heat energy and particles contract back together. • Condensing Point: the temperature at which a gas becomes a liquid due to a loss of heat energy. (physical property)

Sublimation • The physical change of a solid directly to a gaseous phase without first going through the liquid phase - Ex: snow and dry ice

Deposition Occurs when a gas or vapor changes directly into a solid without first going through the liquid phase. Example: Frost on cars

Things to Remember… • The arrangement of molecules in substances become less orderly as they melt and more orderly as they freeze. • The temperature of a substance does not change during a phase change. • The temperatures at which substances changes phases are different for different substances and the same for the same substances.
